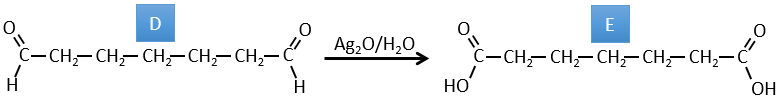Cette section fait partie intégrante du cours et contient quelques réactions qui n’ont pas été abordées dans le cours principal. Il est difficile de comprendre par vous-même certains mécanismes avec les conseils qui sont dans les exercices. Si vous ne réussissez pas, ne vous inquiétez pas. Les solutions et la description des réactions suivent directement chaque exercice. Cependant, les parties qui ont été vues dans le cours sont considérées comme connues et ne peuvent pas être expliquées en détail.
Le principe des exercices suivants est assez simple (mais les exercices ne le sont pas): Nous vous donnons une série de réactions qui se suivent mais nous ne vous donnerons que quelques-uns des réactifs et/ou des produits. De là, vous devez reconstruire tout le processus et découvrir quelle molécule et quelle structure se cachent derrière cette lettre.
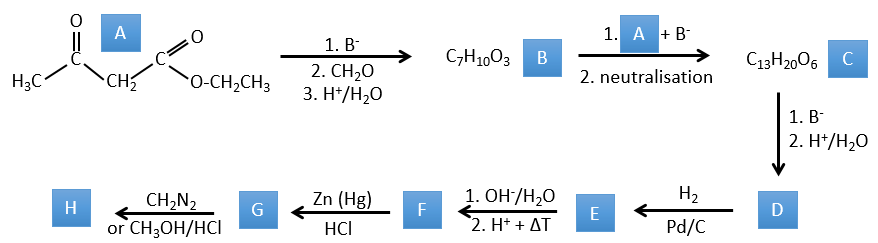
1. Dans ce premier exercice, vous avez la structure du réactif initial (A) et la composition du premier produit B. Vous devez déterminer la structure de B, le produit obtenu par la première réaction. Ensuite, à partir de B, vous devez déterminer la structure de C, puis le composé D et sa structure, etc.
Correction
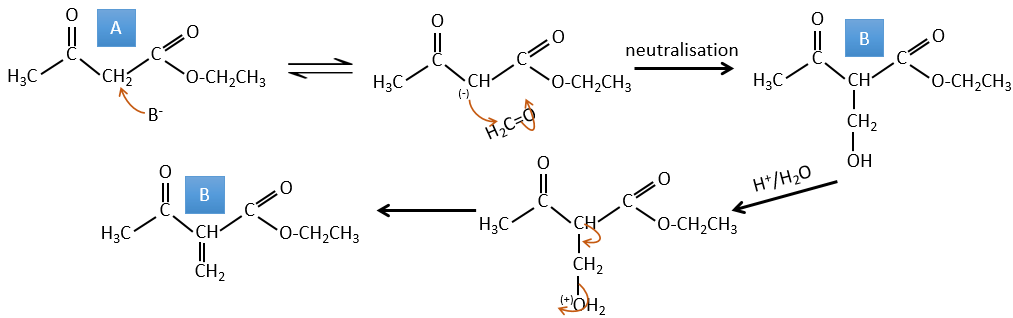
A → B: Deux ensembles de protons sont plus acides que les autres: les protons en α de carbonyle. Les protons de CH3 sont moins acides que le CH2 parce que CH2 est entre deux carbonyles. C’est là que la base attaque. Le carbone négatif attaque ensuite le formaldéhyde. Un réarrangement se produit après la neutralisation pour obtenir B et H2O.
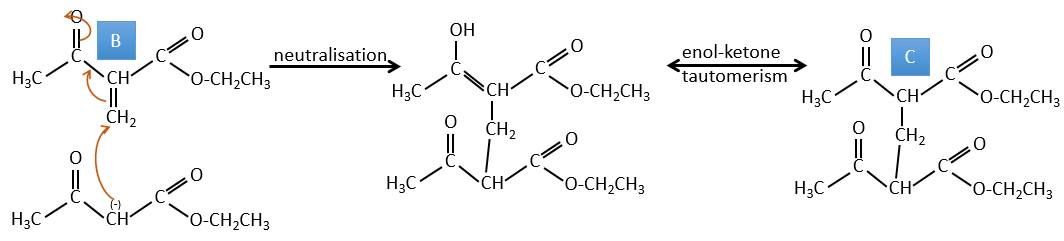
B → C: La première étape est identique à celle de la réaction précédente et les deux molécules se confondent. L’attaque se fait sur le carbone sp2 et on obtient une structure stabilisée par une liaison H formant un cycle entre l’OH et le C = O.
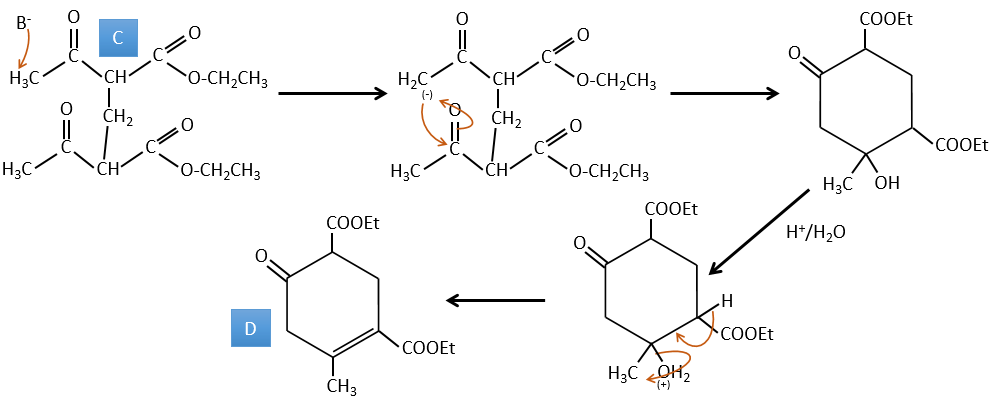
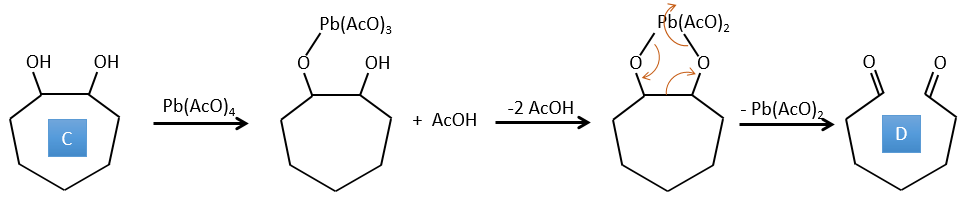
C → D: Cette fois, le proton (noté *) n’est pas attaqué par la base parce que l’élimination d’un proton méthylique permet la formation d’un cycle de 6 carbones. Dans la seconde étape, l’acide catalyse la perte d’une molécule d’eau pour obtenir une double liaison conjuguée au carbonyle.
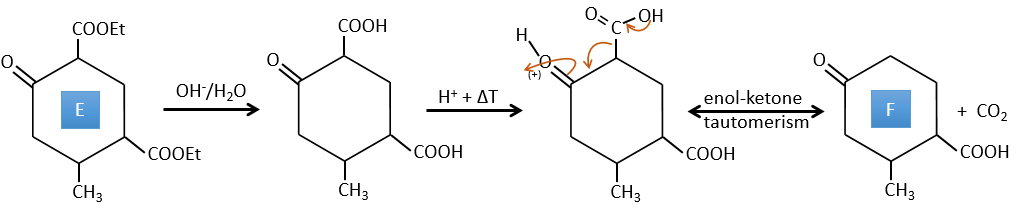
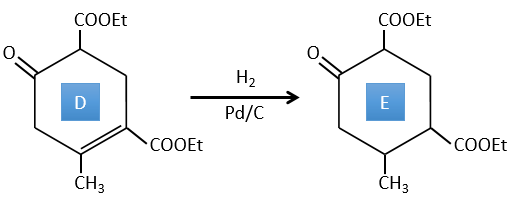
D → E: La double liaison est réduite pour obtenir un cyclohexane.
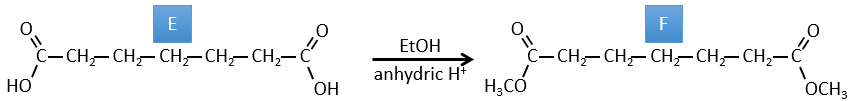
E→F:Les esters sont transformés en acides carboxyliques avec une catalyse basique. Le CO2 se détachera si on augmente la température.
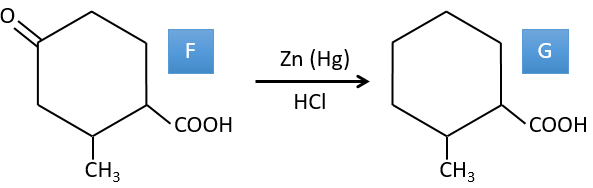
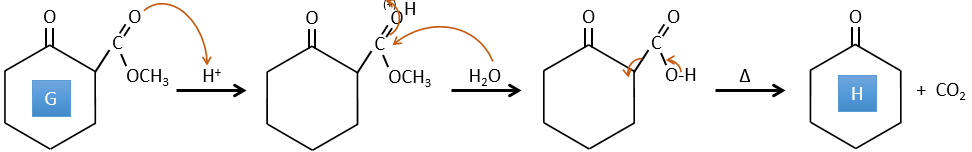
F→G: le Zn réduit sélectivement une cétone dans une chaîne alkyle.
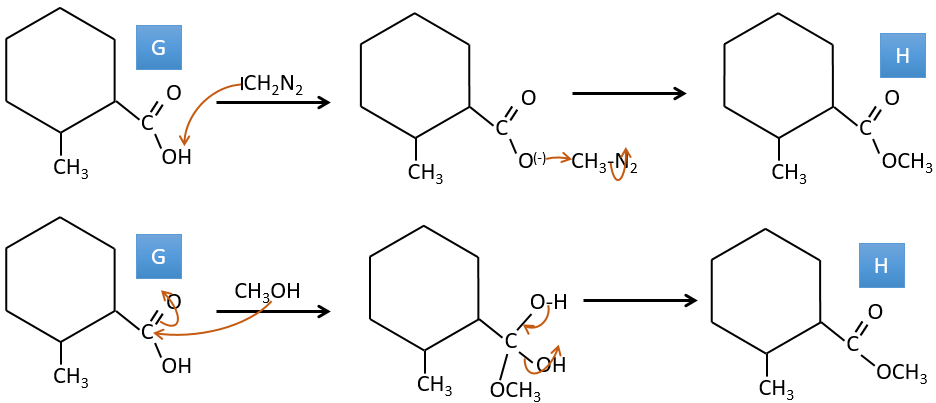
G→H: Le diazonium carbène est capable de prendre le proton de l’acide. Le carboxylate attaque ensuite le carbène pour former un ester méthylique et libérer N2. CH3OH conduit au même résultat mais le processus est différent: il y a une substitution nucléophile sur le carbonyle pour remplacer -OH par
-OCH3.
2. Cet exercice met l’accent sur les réactions concernant les produits aromatiques et les acides carboxyliques ainsi que leurs dérivés.
Correction
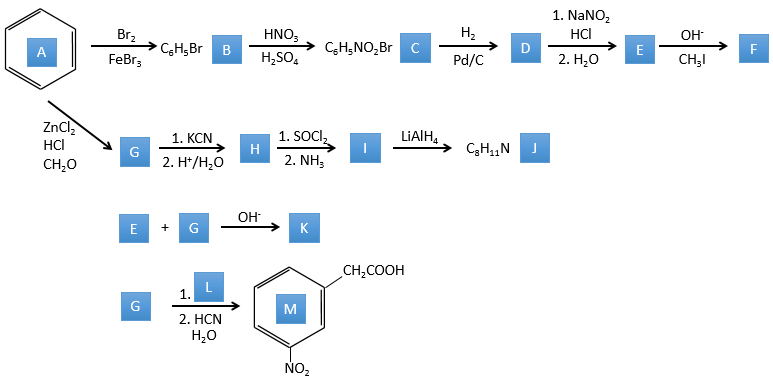
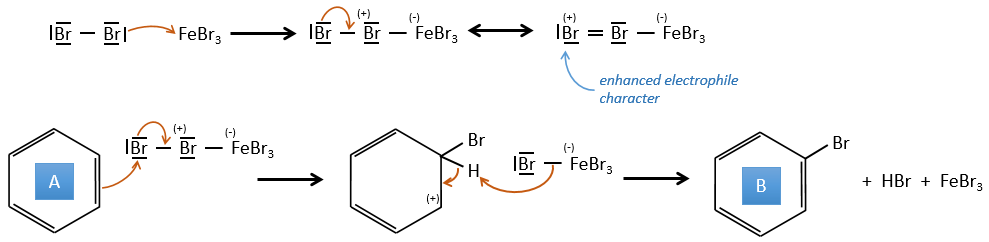
A→B: Le caractère électrophile de Br doit être renforcé par un acide de Lewis pour effectuer la réaction.
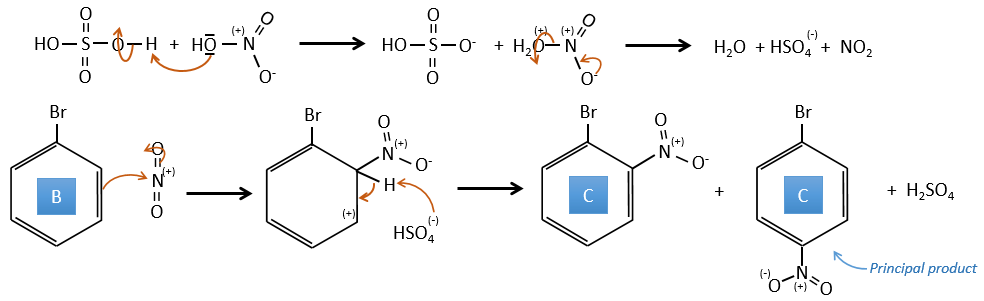
B→C: Un groupe nitro est ajouté au cycle. Il ya déjà un substituant sur le cycle donc nous devons déterminer si le groupe nitro est ajouté en ortho, méta ou para. Un halogène oriente la réaction sur les positions ortho / para. La position para doit être favorisée en raison de l’empêchement stérique sur la position ortho.
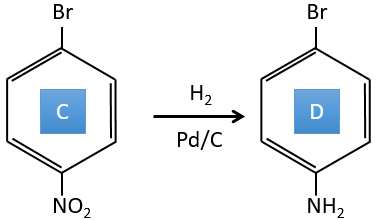
C→D: La réduction est limitée au groupe nitro qui se transforme en amine. Pour réduire complètement le cycle aromatique, nous devons chauffer la solution à 300 ° C.
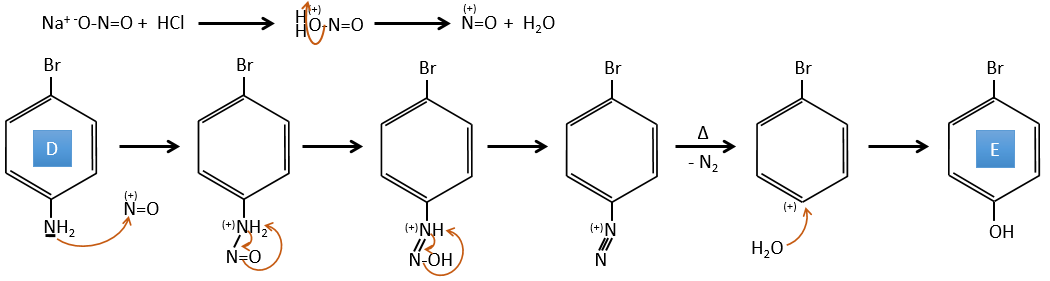
D→E: NaNO2 n’ajoute pas un groupe nitro sur l’anneau. Il conduit à l’élimination de l’aminé et à la formation d’un arénium. Cette espèce très réactive réagit avec l’eau pour remplacer l’aminé par un groupe hydroxyle -OH.
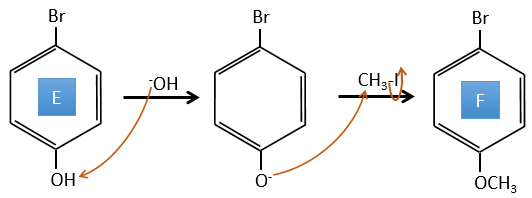
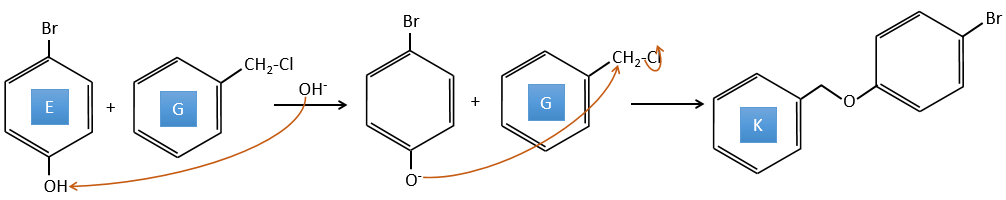
E→F: La base prend le proton du p-bromophénol. Il y a alors une attaque nucléophile par l’anion sur CH3I pour obtenir le p-bromométhoxybenzène.
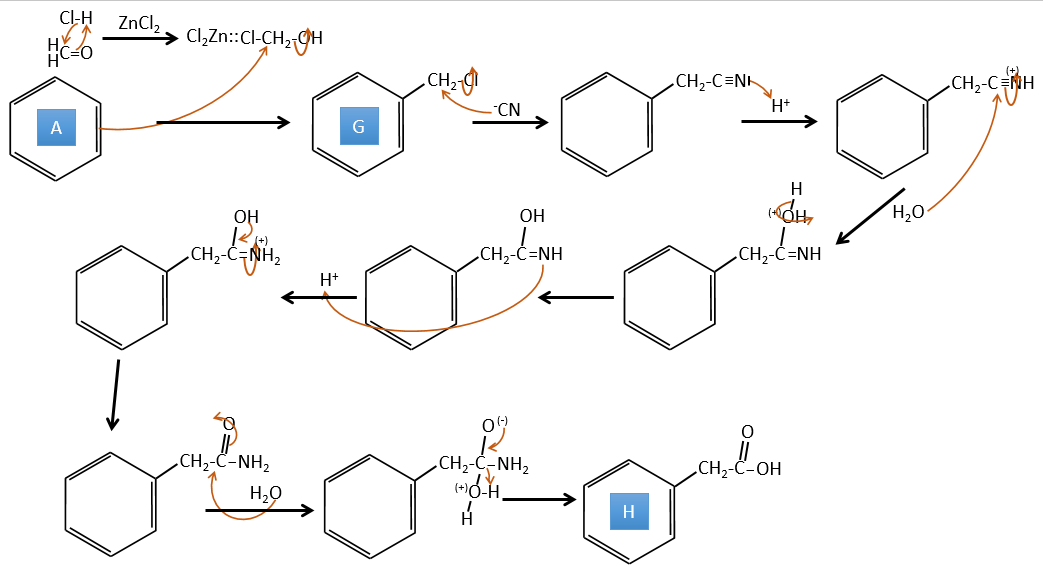
A→G: It is the chloromethylation reaction. During this reaction, the formaldehyde and the chlorydric acid form a chloromethanol stabilised by ZnCl2. The acid protonates the alcohol and the ring can attack it to reject water and bind CH2Cl.
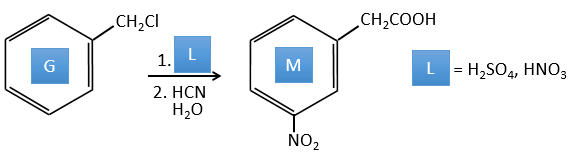
G→H: A simple SN2 by CN– followed by its transformation into a carboxylic acid. This transformation is done by successive attacks of water molecules on the carbon bond to the nitrogen.
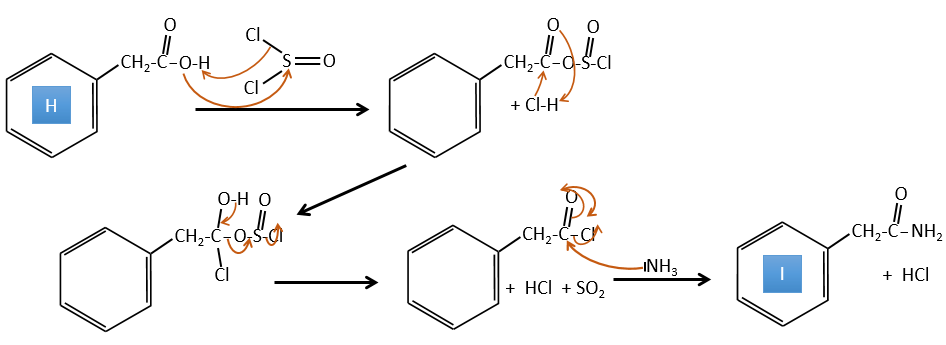
H→I: SOCl2 is a molecule that allows us to obtain an acyl chloride from an acid. It cannot be done with HCl or Cl2 because Cl– is a better leaving group than OH–. The reaction is followed by the formation of a primary amide.
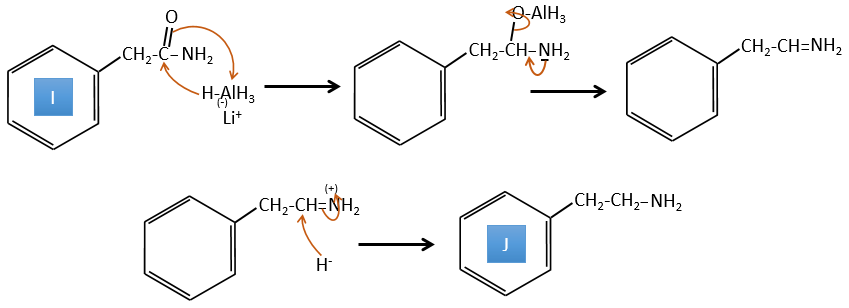
I→J: The amide is reduce into an amine by LiAlH4. LiAlH4 can generates H– that attacks the carbonyl.
E+G→K: A base takes the proton from the bromophenol to obtain a stronger nucleophile. A SN2 takes place between the two species to merge them into one molecule.
G→M: The second step of the reaction leads to the formation of the carboxylic acid as it was the case in the reaction G->H. The missing element on M is the nitro group in meta. This position is favoured because of the mesomeric captor effect of the COOH through the CH2. The effect is however smaller than for a mesomeric captor directly in contact with the aromatic ring.
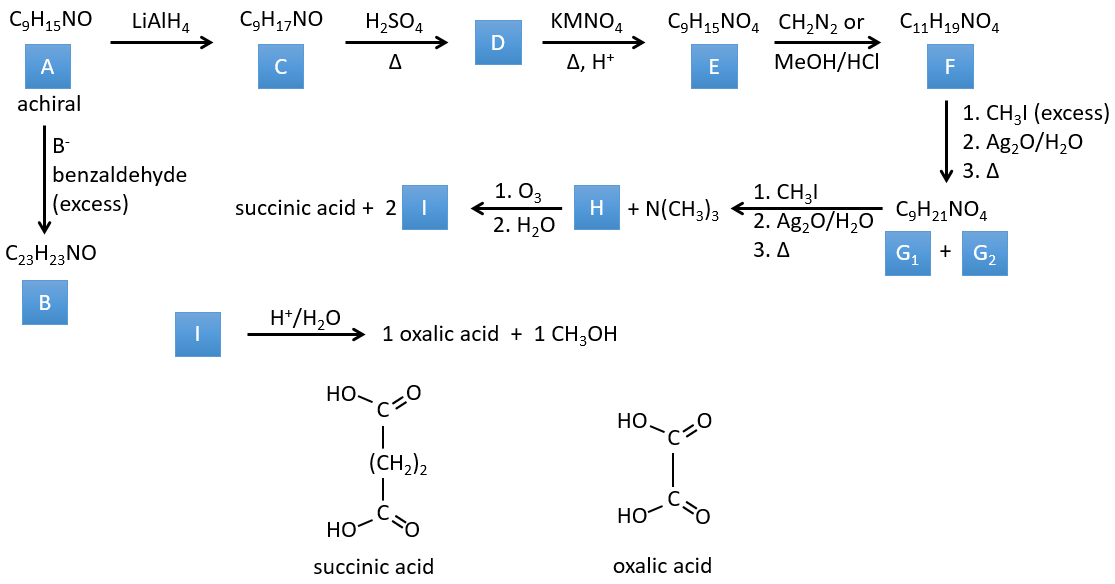
3. In this exercise the last product of a long series of reaction is given. It is the direct product of a reaction of ozonolysis. You have thus to go backwards in the reactions, starting from the end to find the reactants of each reaction. The formulas of most of the molecules are given. G1 and G2 are isomers.
Correction
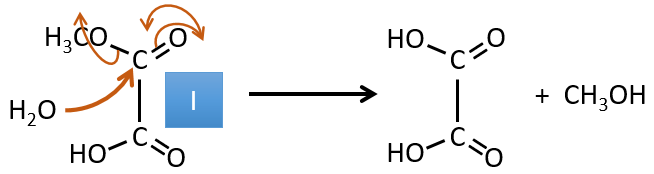
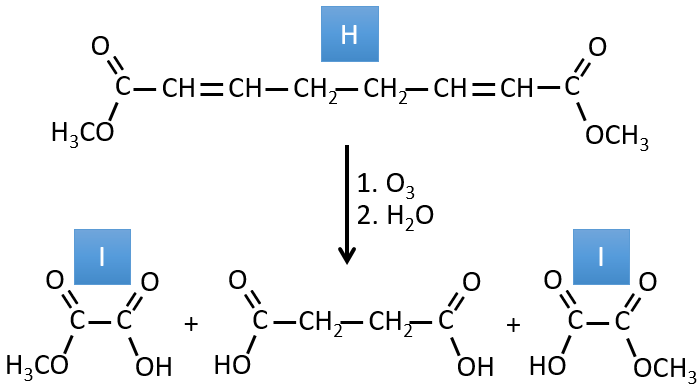
I→…: One of the products, the oxalic acid, is a carboxylic acid and one reactant is the water. We can thus guess that the reaction is a reaction of substitution on a derivative of carboxylic acid. The other product of the reaction is one methanol molecule. One carboxylic acid was thus an ester before the reaction. Only one methanol is generated by the reaction so only one of the two acids of the oxalic acid was an ester.
H→I: The ozonolysis cuts a molecule at double liaisons and leads to the formation of carboxylic acids in presence of an oxidant. There are 4 acid groups in the products of this reaction and 2 esters. The 4 acid groups indicate that a bigger molecule was cut down at two places. The double liaisons were thus conjugated with the esters. It is thus an example of reaction that involves a part of the conjugated system and not all of it. We don’t know if the double liaisons are cis or trans.
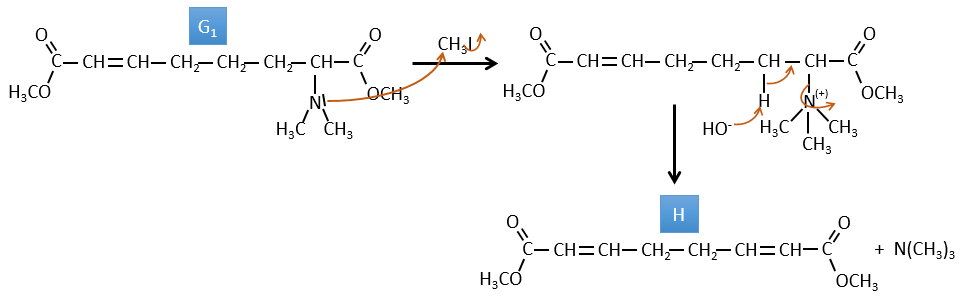
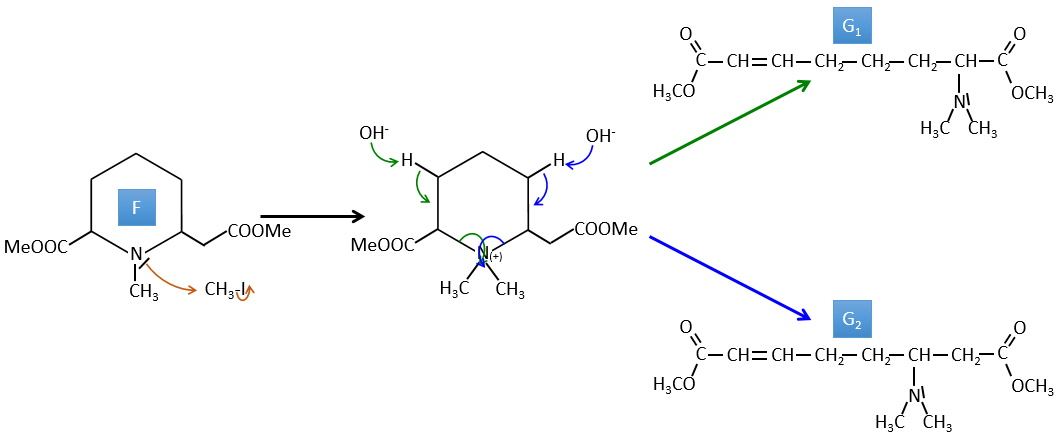
G→H: G1 and G2 are two isomers. There are two other information that we may consider to find the isomers. CH3I, Ag2O and delta are the reactants of the Hoffmann reaction. This reaction breaks one C-N bond and forms a double liaison on this carbon. It is thus one of the pi liaisons that the nitrogen was bound. The second information is that the nitrogen is no more on the product, meaning that it had only one liaison with the chain. The second product of the reaction, N(CH3)3, confirms that. The two isomers are thus different from the carbon on which N(CH3)2 was bound. It can be the carbon in α or in β of carbonyl.
F→G: It is the same reaction than G->H but the nitrogen is still on the molecule after the reaction. It means that it was bound somewhere else on the molecule. The location is where the pi liaison stands. The molecule had thus a cycle of 6 atoms prior to the reaction. Contrarily to the species H, the cycle F is not symmetric. It is why we can obtain two isomers G1 and G2.
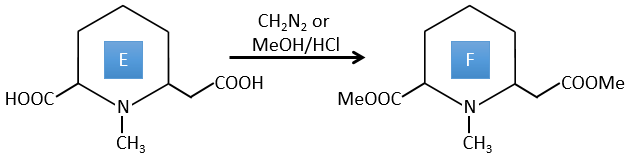
E→F: CH2N2 and MeOH/HCl are two techniques to replace a carboxylic acid by a methylic ester. The species E has thus two carboxylic acids.
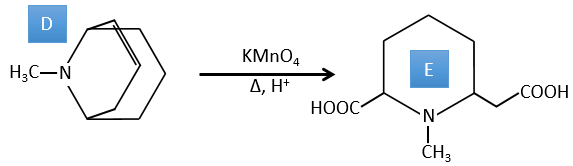
D→E: KMnO4 acts like the ozone. The two carboxylic acids were thus forming one bridge of the cycle. This bridge also forms a cycle of 6 atoms at the side of the nitrogen (and a cycle of 8 carbons with the other side).
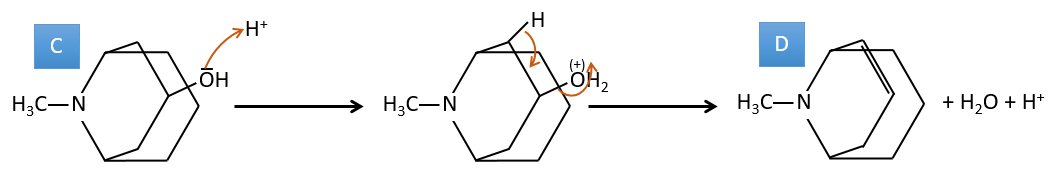
C→D: If we check the compositions of the reactant C and of the product D, we see that there is a difference of H2O. The role of H2SO4 was thus to remove this molecule of water from C with the formation of a double liaison. The hydroxyl group could be at two places (in α or β of the bridged carbons). At this point, we cannot say which position is correct but the reaction A->B is only possible with the hydroxyl group in β of the bridged carbon. The species is thus symmetric and achiral as is the species A.
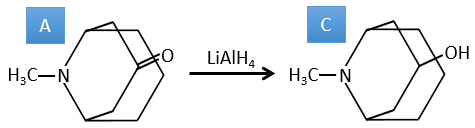
A→C: A classic reaction of reduction. A gets H2 in the process and we can assume that the hydroxyl group was a ketone before the reduction.
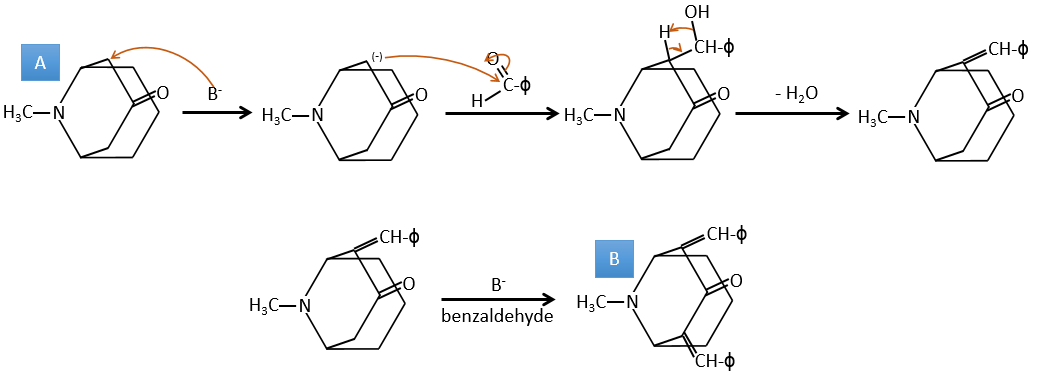
A→B: The base is there to remove a proton in α of carbonyl. Those protons are acid in reason of the tautomerism enol-ketone. The carbanion attacks one benzaldehyde on its carbonyl and water is lost after this attack. The double liaison is in α of carbonyl and forms a long resonance chain with the phenyl. This reaction can be repeated on the other side of the carbonyl to obtain the product B.
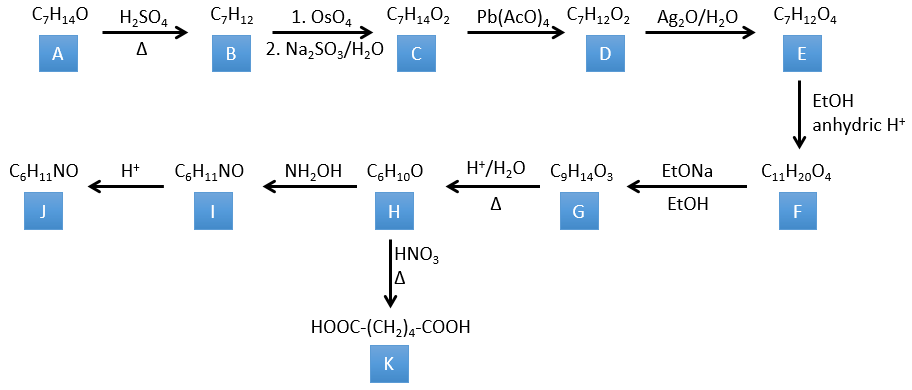
4. There are a few specific reactions in this exercise (mainly BàE). You have the formulas of all the compounds but only the structure of the compound K to start with. HNO3, ΔT is a reactant that breaks the C-C liaison of a ketone to obtain two carboxylic acids. The mechanism is unknown.
Correction
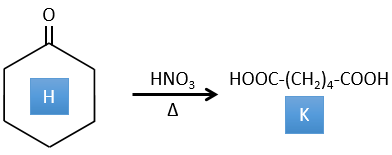
H→K: As explained in the wording, HNO3 breaks a ketone into two acids. As the two acids are present in the product, H is a cycle with a ketone on it. The cycle has 6 carbons.
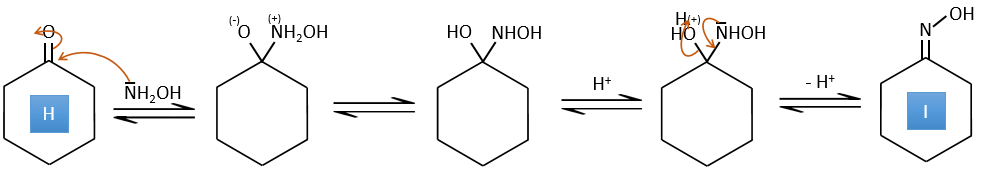
H→I: This reaction leads to the formation of an oxime, i.e. a base of Schiff where R=OH. The mechanism involves a nucleophilic substitution by the nitrogen on the carbonyl to reject one water molecule.
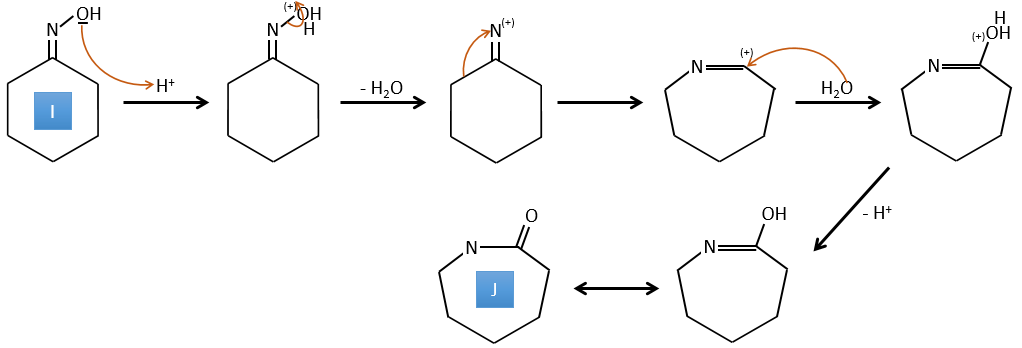
I→J: This reaction is the transposition of Beckmann. Under acid conditions, the OH of the oxime is protonated and water is freed. The cationic nitrogen is attacked next by a carbon in alpha of the oxime, placing the nitrogen into the chain. The water comes back to attack the carbocation and to form an amide.
G→H: A simple reaction to change an ester into a carboxylic acid. The group is then removed from the molecule by an elevation of temperature.
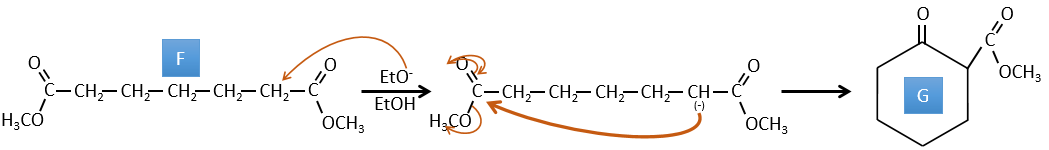
F→G: A proton in α on carbonyl is taken by the strong base. The formed anion attacks next the ester to form a cycle of 6 carbons wearing one ketone and one methylic ester.
E→F: The carboxylic acids are replaced by methylic esters.
D→E: Ag2O is able to oxidize an aldehyde into a carboxylic acid.
C→D: Pb(AcO)4 is a compound that reacts with cis-glycols. Pb exchanges one equivalent of acetic acid to bind with one oxygen. The process is slow but it will also bind with the second OH to form a cycle of 5 atoms. This cycle breaks to generate 2 ketones that are now separated. The same mechanism is obtained with HIO4. As there is only one product, the he reactant is thus a cyclic cis-glycol.
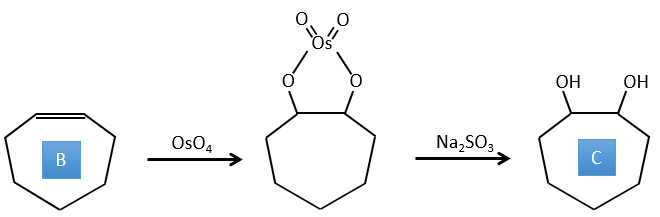
B→C: The osmium tetroxide is a reactant that affects specifically C=C and that generates an osmic ester, a bit like the complex formed by Pb(AcO)4 with the cis-glycol. Na2SO3/H2O removes the osmium from the molecule to obtain the cis-diol. KMnO4 can do the same but we have to be in basic and cold conditions or we will obtain a diacid. The reactant is thus a cycle of 7 carbon with a double liaison between two of the carbons.
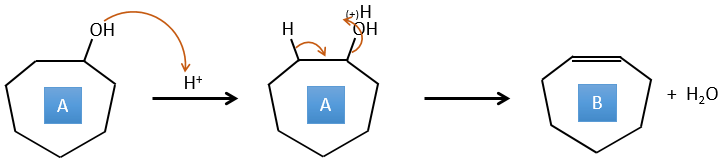
A→B: This reaction is simply the removal of a molecule of water from the cycle that gives a double liaison. We can deduce this from the difference of composition between the reactant and the product: C7H14O-C7H12=H2O.