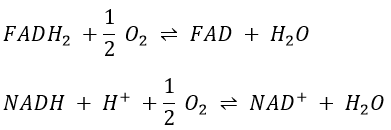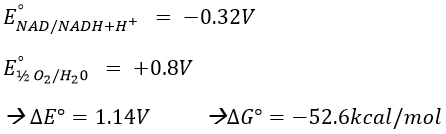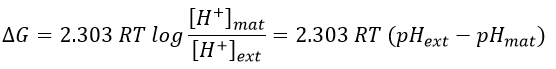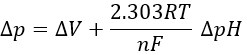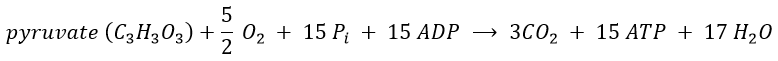Chapter 7b : Glucose catabolism – respiratory chain
In 1935, Engelhardt analysed the rate of ATP in red blood cells (globules rouges) as a function of the rate of oxygen. The experiments showed that the ATP increases with the quantity of O2. Contrarily to the yeast, the blood cells had to be in one piece to observe the phenomenon. Even more, some enzymes contained in an extract of blood cells destroy the ATP. Later, Kalkar showed that if we add one enzyme to the extract the ratio of ATP increases in presence of oxygen.
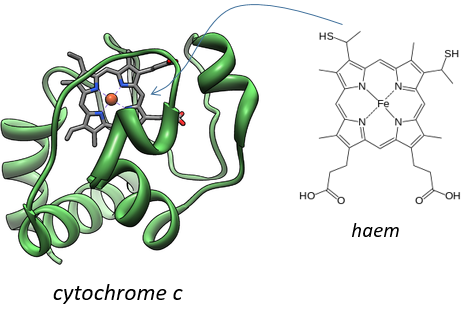
Keilin (1925) was studying the mosquitoes responsible of the malaria. With a spectromicroscope he looked at the legs of one mosquito and saw that they change of colour. At rest the muscles are oxidised but when they are excited the muscles consume the oxygen and are thus less oxidised. The colour is given by the cytochrome, a molecule that changes of colour in function of the oxidation.
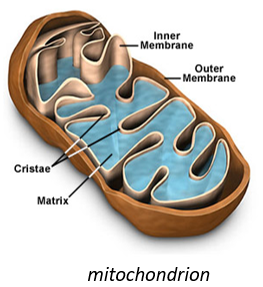
This molecule belongs to the mitochondrion. The mitochondrion is a kind of bacteria hosted by eukaryotes.
They have their own membrane, composed of an inner membrane and of a porous outer membrane. The matrix is inside the inner membrane and the intermembrane space is also called cristae where the membrane is protruding. Similarly to the haemoglobin, the cytochrome is a protein that possesses a haem the iron of which can change of oxidation state. This property is used in the respiratory chain. Cytochrome belongs to the inner membrane of the mitochondrion along which it can move and it separate the glycolysis from the citric acid cycle. The cycle of the citric acid takes place in the matrix of the mitochondrion while glycolysis takes place outside of the mitochondrion.
Respiratory chain
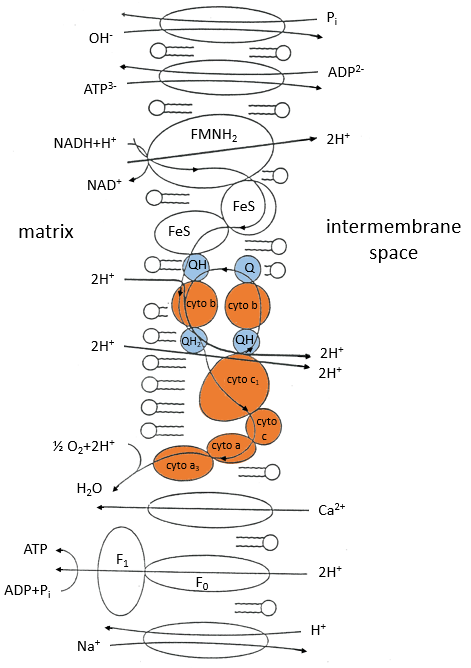
The respiratory chain can be summarised by the following figure and table:
In the inner membrane we find 4 complexes – NADH-ubiquinone reductase, succinate-ubiquinone reductase, ubiquinol-cytochrome c reductase and cytochrome oxidase – that make redox reactions and transfer electrons from one side of the membrane to the other side (sometimes protons instead of electrons). One reaction at one side of the membrane (matrix or intermembrane space) is always coupled with one reaction at the other side of the membrane. One reaction of reduction is coupled with a reduction of oxidation and vice-versa. The whole phenomenon involves transfers of electrons inside the membrane and transfers of protons from one side of the membrane to the other side. Note that some complexes belong to the membrane but only face one of its sides.
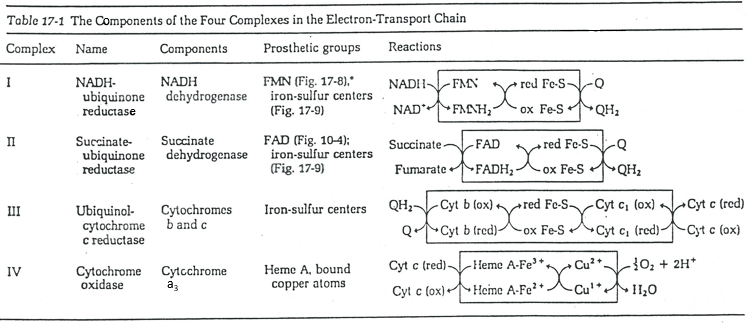
Q is the ubiquinone, a cofactor that belongs to the membrane. It interacts with the cytochrome c in a loop of oxidoreduction. One can see that the oxygen is involved by the complex IV. To resume the loop, we can write two reactions of oxidoreduction, one with the NAD and one with the FAD.
However O2 cannot directly interact with FADH2 and NADH+H+ and this loop is thus necessary. For redox, the free energy of Gibbs is
To determine the difference of potential ∆E°, we look at the couples involved in the reaction:
For FAD we have 43.3kcal/mol. There is a transfer of protons involved in the process but there must be an equilibrium between the concentrations of charge inside the cell and outside the cell. The protons can move out of the matrix of the mitochondrion through some protein complexes that generate ATP from the flow of protons (bottom of the figure). The F1-ATPase protein is composed of two parts, F0 in the membrane and F1 in the matrix of the mitochondrion. F0 pumps the protons from the intermembrane space towards the matrix. F1 receives the energy from the transport and transforms ADP into ATP. Those species are charged negatively and require a transport through the membrane (top of the figure). One ADP can go in the matrix only if one ATP is moving out of the matrix. The inorganic phosphates Pi are also transported through the membrane.
The energy to form the ATP comes from the gradient of pH. It generates a proton motive force.
Where the indexes mat and ext respectively matrix and its exterior (the intermembrane space). The gradient of pH generates a difference of electric potential ΔV.
Combining the two effects, we have
Dividing this expression by nF, we obtain the expression of a variation of free energy ∆p associated to the transfer of one mole of protons (in volt).
The coefficient 2.303RT/nF of the gradient of pH has a value of 59mV at 25°C.
We can measure the pH and the difference of potential: ∆V=0.14V and ∆pH=-1.4.
It corresponds to a variation of free energy equal to
To form one mole of ATP, we need 7.3kcal. In the respiratory chain, there are 4 complexes that oxidise the NADH and the FAD. They respectively form the equivalent of 11 and 8 protons by loop.
We can write a global equation of the “combustion” of a pyruvate during the citric acid cycle and the respiratory chain:To summarise, the oxidation of one NADH generates ~3 ATP and the oxidation of FAD generates ~2ATP.
The 15 ATP come from
- 4 NADH (one before the citric acid cycle and 3 during the cycle) à12ATP,
- from 1 FADH2 à 2ATP
- and from 1 GTP»1 ATP.
To form one pyruvate, glycolysis forms 2 ATP and uses 2 NAD that are reduced into NADH+H+.
So considering 3 ATP by NADH, it corresponds to the production of 8 ATP. Added to the 15 ATP of each pyruvate (x2), we obtain 38ATP by glucose. In comparison to the combustion of the glucose (∆G°’=-686kcal/mol), it represents about 40% of the potential energy (38×7.3kcal/mol=277kcal/mol).
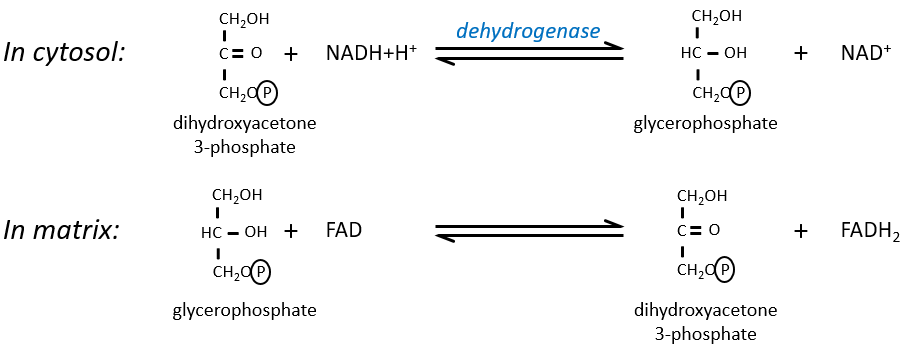
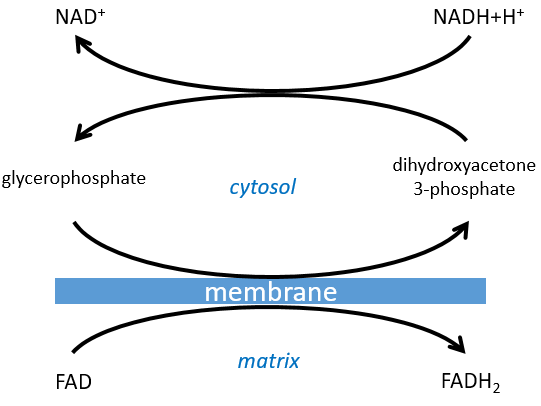
The NAD involved in the glycolysis and that produces a NADH is not at the same place (cytosol) that the NADH required in the cellular respiration (matrix of the mitochondrion). The NADH cannot enter freely into the mitochondrion because it is charged. There is a system of shuttle that may differ for different cells and that will modify the NADH to allow its passage through the membrane, then modify it again so it can be used there. In the brain and in muscles, it is a shuttle of glycophosphate that we will next explain. The shuttle is basically composed of two reactions: one in the cytosol and one in the matrix of the mitochondrion:
The shuttle can be represented like this:
If you remember, dihydroxyacetone-3P was part of the glycolysis (red rectangle in the following figure): it is one of the two trioses phosphate produced from fructose -1,6-diphosphate. The fact that this triose is involved in the shuttle does not really decrease the yield of the glycolysis significantly: the cell needs a given quantity of dihydroxyacetone for the shuttle but the molecules are regenerated in the matrix of mitochondria and can then return to the cytosol. So they just have to be produced once. The rest of the production is turned into glyceraldehyde 3P for the glycolysis.
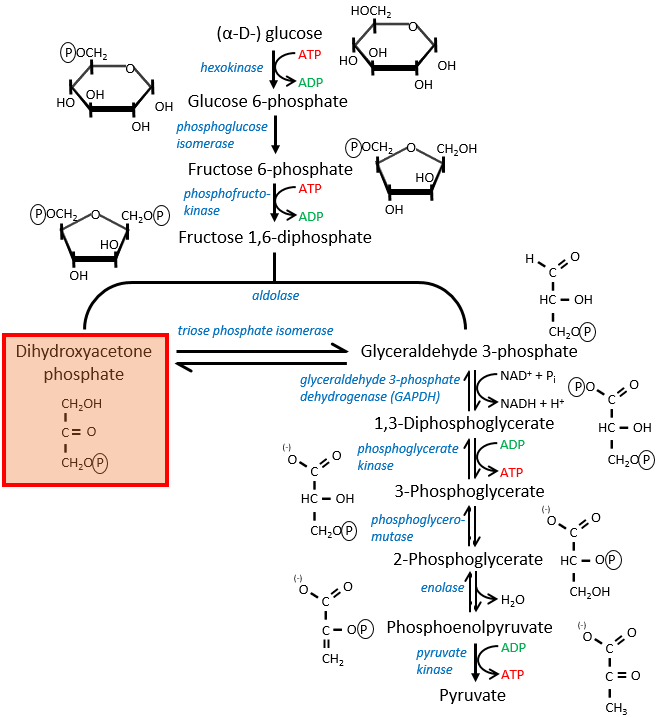
The FADH2 is oxidised during the respiration and the dihydroxyacetone can move back to the cytosol. The shuttle has a small cost: it oxidises one NADH into NAD in the cytosol while the reaction in the matrix involves FAD/FADH2. In term of energy, it represents one ATP used because all the processes don’t take place at the same place.
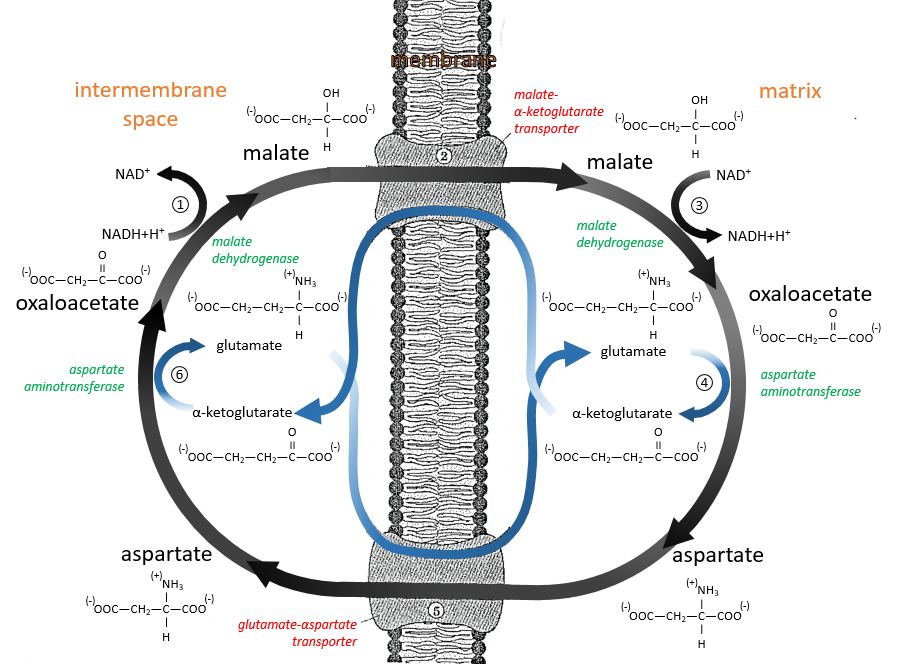
The malate-aspartate shuttle
The same cofactors (NADH+H+/NAD+) are used so the yield does not change with this shuttle (38 ATP). The mechanism is based on the reducing power of the oxaloacetate.
Reduced into malate (1), it is transported in the matrix of the mitochondrion (2) through the malate-α-ketoglutarate transporter if one α-ketoglutarate is available to make the displacement in the opposite direction. The reverse reaction, the oxidation of the malate into the oxaloacetate is made in the matrix (3). Yet, the oxaloacetate cannot goes back out of the membrane. To do so, it is transformed into an amino acid, the aspartate, by a reaction of transamination (4):
It is done here by the aspartate transaminase with the use of the glutamate as amino acid. As a result, the oxaloacetate is transformed into aspartate that can move through the membrane through a transporter requiring the passage in the opposite direction of a glutamate (5). In the cytosol, it is turned back into the oxaloacetate with the exact same reaction (6) than at the other side of the membrane.