Chapter 10 : meiosis
The sequence of events during meiosis involves two nuclear divisions ( meiosis I and meiosis II ).
Prophase 1
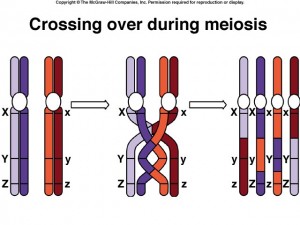
In prophase 1 of meiosis, the DNA coils tighter, and individual chromosomes first become visible under the light microscope as a matrix of fine threads. Because the DNA has already replicated before the onset of meiosis, each of these threads actually consist of two sister chromatids joined at their centromeres. In prophase 1, homologous chromosomes become closely associated in synapsis, exchange segments by crossing over, and then separate.
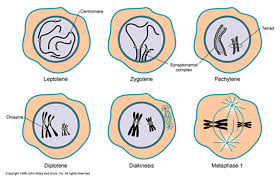
Prophase 1 is traditionally divided into five sequential stages: leptotene, zygotene, pachytene, diplotene, and diakinesis.
Leptotene: during which each chromosome becomes visible as two fine threads (chromatids)
Zygotene: A lattice of protein is laid down between the homologous chromosomes in the process of synapsis, forming a structure called a synaptonemal complex.
Pachytene: Pachytene begins when synapsis is complete (just after the synaptonemal complex forms and lasts for days. This complex, about 100 nm across, holds the two replicated chromosomes in precise register, keeping each gene directly across from its partner on the homologous chromosome. Within the synaptonemal complex, the DNA duplex unwind at certain sites, and single strands of DNA from base-pairs with complementary strands on the other homologue. The synaptonemal complex thus provides the structural framework that enables crossing over between the homologues chromosomes. As you will see, this has a key impact on how the homologues separate later in meiosis.
Diplotene: the fourth stage of the prophase I of meiosis, following pachytene, during which the paired chromosomes begin to seperate (the synaptonemal complex disassembles) into two pairs of chromatids.Diplotene is a period of intense cell growth. During this period the chromosomes decondense and become very active in transcription
Diakinesis: At the beginning of diakinesis, the transition into metaphase, transcription ceases and the chromosomes recondense.
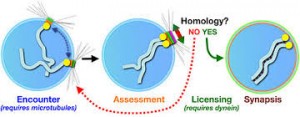
Synapsis: During prophase, the ends of the chromatids attach to the nuclear envelope at specific sites. The sites the homologous attach to are adjacent, so that the members of each homologous pair of chromosomes are brought close together. They then line up side by side, apparently guided by heterochromatin sequences, in the process called synapsis.
Crossing Over
Within the synaptonemal complex, recombination is thought to be carried out during pachytene by very large protein assemblies called recombination nodules. A nodule’s diameter is about 90 nm, spanning the central element of the synaptonemal complex. The details of the crossing over process are not well understood, but involve a complex series of events in which DNA segments are exchanged between nonsister or sister chromatids. In humans, an average of two or three such crossover events occur per chromosome pair.
When crossing over is complete, the synaptonemal complex breaks down, and the homologous chromosomes are released from the nuclear envelope and begin to move away from each other. At this point, there are four chromatids for each other. At this point, there are four chromatids for each type of chromosome (two homologous chromosomes, each of which consists of two sister chromatids). The four chromatids do not separate completely, however, because they are held together in two ways: (1) the two sister chromatids of each homologue, recently created by DNA replication, are held near by their common centromeres; and (2) the paired homologues are held together at the points where crossing over occurred within the synaptonemal complex.
Chiasma Formation
Evidence of crossing over can often be seen under the light microscope as an X-shaped structure known as a chiasma. The presence of a chiasma indicates that two chromatids (one from cach homologue) have exchanged parts. Like small rings moving down two strands of rope, the chiasmata move to the end of the chromosome arm as the homologous chromosomes separate.
Synapsis is the close pairing of homologous chromosomes that takes place early in prophase 1 of meiosis. Crossing over occurs between the paired DNA strands, creating the chromosomal configurations known as chiasmata. The two homologues are locked together by these exchanges and they do not disengage readily.
Metaphase 1
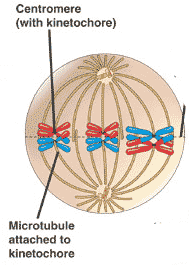
By metaphase 1, the second stage of meiosis 1, the nuclear envelope has dispersed and the microtubules form a spindle, just as in mitosis. During diakinesis of prophase 1, the chiasmata move down the paired chromosomes from their original points of crossing over, eventually reaching the ends of the chromosomes. At this point, they are called terminal chiasmata. Terminal chiasmata hold the homologous chromosomes together in metaphase 1, so that only one side of each centromere faces outward from the complex; the other side is turned inward toward the other homologue. Consequently, spindle microtubules are able to attach to kinetochore proteins only on the outside of each centromere, and the centromeres of the two homologues attach to microtubules originating from opposite poles. This one-sided attachment is in marked contrast to the attachment in mitosis, when kinetochores on both sides of a centromere bind to microtubules.
Each joined pair of homologues then lines up on the metaphase plate. The orientation of each pair on the spindle axis is random: either the maternal or the paternal homologue may orient toward a given pole.
Chiasmata play an important role in aligning the chromosomes on the metaphase plate.
Completing Meiosis
After the long duration of prophase and metaphase, which together make up 90% or more of the time meiosis 1 takes, meiosis 1 rapidly concludes. Anaphase 1 and telophase 1 proceed quickly, followed – without an intervening period of DNA synthesis – by the second meiotic division.
Anaphase 1
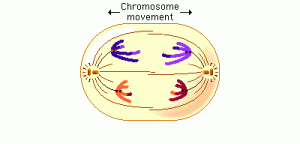
In anaphase 1, the microtubules of the spindle fibers begin to shorten. As they shorten, they break the chiasmata and pull the centromeres toward the poles, dragging the chromosomes along with them. Because the microtubules are attached to kinetochores on only one side of each centromere, the individual centromeres are not pulled apart to form two daughter centromeres, as they are in mitosis. Instead, the entire centromere moves to one pole, taking both sister chromatids with it. When the spindle fibers have fully contracted, each pole has a complete haploid set of chromosomes consisting of one member of each homologous pair. Because of the random orientation of homologous chromosomes on the metaphase plate, a pole may receive either the maternal or the paternal homologue from each chromosome pair. As a result, the genes on different chromosomes assort independently; that is, meiosis 1 result in the independent assortment of maternal and paternal chromosomes into the gametes.
Telophase 1
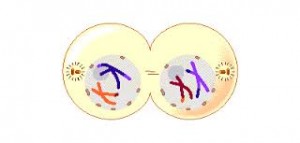
By the beginning of telophase 1, the chromosomes have segregated into two clusters, one at each pole of the cell. Now the nuclear membrane re-forms around each daughter nucleus. Because each chromosome within a daughter nucleus replicated before meiosis 1 began, each now contains two sister chromatids attached by a common centromere. Importantly, the sister chromatids are no longer identical, because of the crossing over that occurred in prophase 1. Cytokinesis may or may not occur after telophase 1. The second meiotic division, meiosis 2, occurs after an interval of variable length.
The Second Meiotic Division
After a typically brief interphase, in which no DNA synthesis occurs, the second meiotic division begins.
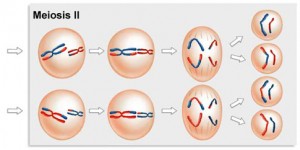
Meiosis 2 resembles a normal mitotic division. Prophase 2, metaphase 2, anaphase 2, and telophase 2 follow in quick succession.
Prophase 2. At the two poles of the cell the clusters of chromosomes enter a brief prophase 2, each nuclear envelope breaking down as a new spindle forms.
Metaphase 2. In metaphase 2, spindle fibers bind to both sides of the centromeres.
Anaphase 2. The spindle fibers contract, splitting the centromeres and moving the sister chromatids to opposite poles.
Telophase 2. Finally, the nuclear envelope re-forms around the four sets of daughter chromosomes.
The final result of this division is four cells containing haploid sets of chromosomes. No two are alike, because of the crossing over in prophase 1. Nuclear envelopes then form around each haploid set of chromosomes. The cells that contain these haploid nuclei may develop directly into gametes, as they do in animals. Alternatively, they may themselves divide mitotically, as they do in plants, fungi, and many protists, eventually producing greater numbers of gametes or, as in the case of some plants and insects, adult individuals of varying ploidy.
During meiosis 1, homologous chromosomes move toward opposite poles in anaphase 1, and individual chromosomes cluster at the two poles in telophase 1. At the end of meiosis 2, each of the four haploid calls contains one copy of every chromosome in the set, rather than two. Because of crossing over, no two cells are the same. These haploid cells may develop directly into gametes, as in animals, or they may divide by mitosis, as in plants, fungi, and many protists.
Chapter 1: Mendelian genetics
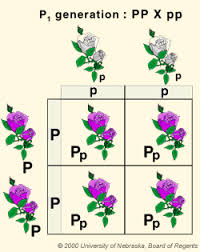
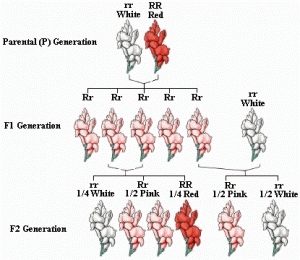
Mendel crossed purple flowers (peas) with white flowers (peas) and he got purple flowers for all of the first generation. Now we should know that the first cross in any genetic cross is called the P cross or the parental cross and then the offspring’s of that are called the F1 or filial 1. When he crossed these purple flowers with themselves, what he got was a 3 to 1 ratio of purple to white flowers peas (just as a crystal white as that first white was in the beginning). And so what he said was that there was a character or trait that was passed through, and for purple flowers this character could be PP and for the white flowers it could be pp so all of F1 generation will be Pp .
1)Segregation:If we have a gene type Pp the probability of P an p is 50%. This separation of genes is called segregation. (random chance of having P or p)
2) Independent assortment: That means the separation of genotypes do not influence each other, so they are independent (But sometimes it is not true because two genes are at same chromosomes).
Problems:
1) A coin is flipped 4 times and comes up heads each time. What is the probability that the next coin flip will come up heads?
2) Classify the following as heterozygous or homozygous: RR, Rr, YY, Yy RR
3)What is the phenotype of the following Yy, Rr, yy, YyRr
4)What is the probability of Rr X Rr producing wrinkled seeds?
5)What is the probability of Yy X yy producing green seeds?
6)What is the probability that Rr Yy X RR Yy would produce Rr Yy?
Punnett square
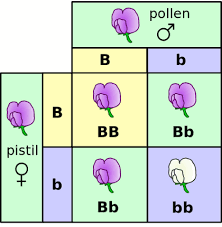
The Punnett square is often over used as just a quick way to solve genetic problems without really understanding it. What’s ‘going on with the genetics? The two sides of Punnett square represent the alternatives after meiosis. In other words you have a brunch of genes and you give half of those genes to a sperm or an egg. And that happens trough meiosis and so the organization of those gametes. In this case it’s just a monohybrid cross, are going to be on either side of this, just like a flip of a coin. This would be for one parent. And then this would be the other parent on the other side. And so what are in boxes on a Punnett square stand for? They simply stand for all the alternatives that could occur if we had mating between each of these different gametes. And so let’s get to some examples and hopefully that will help. So, we are going to start with a monohybrid cross. A monohybrid cross is simply a cross that is looking at one trait. And so let’s say were crossing purple flowers that are homozygous purple with those that are homozygous white flowers. In other words this is the dominant trait and the other one is a recessive trait. And so if you look, at the parents what you Want to do first of all, is figure out what are the possible gametes. That could be produced in meiosis. What does it mean? It can only give one thing, it can only give a big P or that dominant allele. And so if you’re doing a problem like this you don’t need a big Punnett square. The other parent is pp and he can give only a p because the p’s are the same.
We see that there are two possibilities for each parent. And we have to show both of those possible gametes of meiosis. There is a 1 to 2 genotypic ratio PP,pp-homo and Pp,Pp-hetero But a 3 to 1 ratio for phenotype, that means three purple to 1 white. Now let’s see an incomplete dominance; a snapdragon would be an example of that. A snapdragon has two genes; if it has a red gene and a white gene, then it’s going to be pink. And so this one, in this case we will have 1 to 2 ratio in genotype but also in phenotype (co dominance or incomplete dominance)
And now we will try with a sex linked chromosomes or a x linked chromosomes. Ex: is a color blindness gene and the father is normal.
| X | Y | |
| X | XX | XY |
She is not color blind because she has an efficient gene and she is only carrier. So we have a color blind male and a carrier female, a normal female and a normal male.
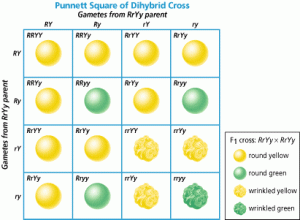
Now let’s have a look on dihybrid cross Rr Yy Rr Yy, R= round pea seeds Y=yellow seeds If it is recessive that stands for wrinkled and if it is a little y that stands for green and so we are looking at a dihybrid, so that means two traits. We’re looking at seed’s shape (round or wrinkled). And seed’s color, yellow or green. So now we have to do a dihybrid cross. And so the tendency is we look at this parent. We can see that there are four letters and there are four boxes and then you just simply write them out. Big R little r, big Y little y and then you get a weird answer and you don’t know what to do with it. Rr Yy What are possibility of gametes? RY, Ry, rY, ry We can do the same with other parent
These nine will be round and yellow, because they are dominant and when we have dominant genotype the phenotype will be the same (round and yellow); four will be (wrinkled and yellow) and one will be green and wrinkled.
Human genetic disorders: Human genetic disorders caused by non-disjunction: Trisomy 21 (Down’s syndrome) ,klinefelters’s syndrome , Xxx metafemals syndrome. Can we survive with only one x or y chromosome? so we will study specific diseases
1) Sickle cell anemia which is a genetic blood disorder (a base substation in a single point). It causes red blood cells to be misshapen. This abnormality is frequent in Africa and Mediterranean regions and in south and central America (where malaria have been prevalent) The alleles for this trait exhibit co-dominance Bn=normal Bs=sickle
| genotype | phenotype |
| BnBn | N |
| BnBs | Normal and sickle cells(sickle trait) |
| BsBs | Sickle cell anemia |
With sickle trait the people have no abnormal episode and it is a good defense against malaria.
2) Huntington’s disease, a genetic disorder du to an abnormal gene on chromosome n° 4. The faulty gene that causes Huntington’s disease is found ( a normal copy of the gene produces huntingtin, a protein. The faulty gene is larger than it should be and produces a larger form of huntingtin)
Some of our brain cells are sensitive to the larger form of huntingtin – it undermines their function and eventually destroys them. Scientists are not sure exactly how this happens. Scientists have figured out why a faulty protein accumulates in cells everywhere in the bodies of people with Huntington’s disease, but only kills cells in the part of the brain that controls movement, causing negligible damage to tissues elsewhere. Huntington disease is unique because it is a dominant gene (dominant genes don’t have to be common in fact and Huntington is really rare ) Why this disease is interesting? If the gene is dominant and the patient dye, how can he passe the gene to the others? The Huntington disease dose not become symptomatique until age 40; so the patient will have time to passe the gene.
3) Hemophilia: The gene for Haemophilia is carried on the X sex chromosome (females are XX; males are XY). A man with Haemophilia passes the faulty gene to his daughters who then carry this gene and may pass the condition onto their sons. A man with Haemophilia does not pass the faulty gene onto his sons because they receive a copy of his Y chromosome (their X chromosome comes from the mother).
Men are affected because the faulty gene on their X chromosome lacks the necessary instructions to produce the clotting factor which causes blood to clot. Women are usually unaffected because they have two X chromosomes and the working copy of the gene tends to override the faulty copy. However, about a third of female carriers have low clotting factor levels and may be mildly affected.
If a carrier mother decides to have children, all her daughters have a 50% (1 in 2) chance of being a carrier. This depends on whether they receive the X chromosome containing the faulty or the working gene. Sons also have a 50% (1 in 2) chance of inheriting the faulty or the working gene. If they inherit the faulty gene, they will have Haemophilia as they do not have a second X chromosome to override the faulty copy.
About 30% of people with the condition have no family history of Haemophilia. They are probably affected because of a spontaneous genetic mutation which took place in the egg or sperm before fertilisation.
Chapter 11 : specific features of meiosis
Meiosis has three unique features :
The mechanism of cell division varies in important details in different organism. This is particularly true of chromosomal separation mechanism, which differ substantially in protists and fungi from the process in plants and animals that we will describe here. Meiosis in a diploid organism consists of two rounds of division, mitosis of one. Although meiosis and mitosis have much in common, meiosis has three unique features: synapsis, homologous recombination, and reduction division.
Synapsis :
The first unique feature of meiosis happens early during the first nuclear division. Following chromosome replication, homologous chromosomes, or homologous pair all along their length. The process of forming these complexes of homologous chromosomes is called synapsis.
Homologous Recombination :
The second unique feature of meiosis is that genetic exchange occurs between the homologous chromosomes while they are thus physically joined. The exchange process that occurs between paired chromosomes is called crossing over. Chromosomes are then drawn together along the equatorial plane of the dividing cell; subsequently, homologues are pulled by microtubules toward opposite poles of the cell. When this process is complete, the cluster of chromosomes at each pole contains one of the two homologues of each chromosome. Each pole is haploid, containing half the number of chromosomes present in the original diploid cell. Sister chromatids do not separate from each other in the first nuclear division, so each homologue is still composed of two chromatids.
Reduction Division :
The third unique feature of meiosis is that the chromosomes do not replicate between the two nuclear divisions, so that at the end of meiosis, each cell contains only half the original complement of chromosomes. In most respects, the second meiotic division is identical to a normal mitotic division. However, because of the crossing over that occurred during the first division, the sister chromatids in meiosis 2 are not identical to each other.
Meiosis is a continuous process, but it is most easily studied when we divide it into arbitrary stages. The stages of meiosis are traditionally called meiosis 1 and meiosis 2. Like mitosis, catch stage is subdivided further into prophase, metaphase, anaphase and telophase. In meiosis, however, prophase 2 is more complex than in mitosis. In meiosis, homologous chromosomes become intimately associated and do not replicate between the two nuclear divisions.
The Red Queen Hypothesis. One evolutionary advantage of sex may be that it allow populations to “store” recessive alleles that are currently bad but have promise for reuse at some time in the future. Because populations are constrained by a changing physical and biological environment, selection is constantly acting against such alleles, but in sexual species can never get rid of those sheltered in heterozygotes. The evolution of most sexual species, most of the time, thus manages to keep pace with ever-changing physical and biological constraints. This “treadmill evolution” is sometimes called the “Red Queen hypothesis”, after the Queen of Hearts in Lewis Carroll’s Through the Looking Glass, who tells Alice, “Now, here, you see, it takes all the running you can do, to keep in the same place.”
Miller’s Ratchet. The geneticist Herman Miller pointed out in 1965 that asexual populations incorporate a kind of mutational ratchet mechanism – once harmful mutations arise, asexual populations have no way of eliminating them, and they accumulate over time, like turning a ratchet. Sexual populations, on the other hand, can employ recombination to generate individuals carrying fewer mutations, which selection can then favor. Sex may just be a way to keep the mutational load down.
The Evolutionary Consequences of Sex
While our knowledge of how sex evolved is sketchy, it is abundantly clear that sexual reproduction has an enormous impact on how species evolve today, because of its ability to rapidly generate new genetic combinations. Independent assortment, crossing over, and random fertilization each help generate genetic diversity.
Whatever the forces that led to sexual reproduction, its evolutionary consequences have been profound. No genetic process generates diversity more quickly; and, as you will see in later chapters, genetic diversity is the raw material of evolution, the fuel that drives it and determines its potential directions. In many cases, the pace of evolution appears to increase as the level of genetic diversity increases. Programs for selecting larger stature in domesticated animals such as cattle and sheep, for example, proceed rapidly at first, but then slow as the existing genetic combinations are exhausted; further progress must then await the generation of new gene combinations. Racehorse breeding provides a graphic example: thoroughbred racehorses are all descendants of a small initial number of individuals, and selection for speed has accomplished all it can with this limited amount of genetic variability – the winning times in major races ceased to improve decades ago.
Paradoxically, the evolutionary process is thus both revolutionary process is thus both revolutionary and conservative. It is revolutionary in that the pace of evolutionary change is quickened by genetic recombination, much of which results from sexual reproduction. It is conservative in that evolutionary change is not always favored by selection, which may instead preserve existing combinations of genes. These conservative pressures appear to be greatest in some asexually reproducing organism that do not move around freely and that live in especially demanding habitats. In vertebrates, on the other hand, the evolutionary premium appears to have been on versatility, and sexual reproduction is the predominant mode of reproduction by an overwhelming margin.
The close association between homologous chromosomes that occurs during meiosis may have evolved as mechanism to repair chromosomal damage, although several alternative mechanism have also been proposed.
The evolutionary origin of sex is a puzzle.
Why Sex?
Not all reproduction is sexual. In asexual reproduction, an individual inherits all of its chromosomes from a single parent and is, therefore, genetically identical to its parent. Bacterial cells reproduce asexually, undergoing binary fission to produce two daughter cells containing the same genetic information. Most protists reproduce asexually except under conditions of stress; then they switch to sexual reproduction. Among plants, asexual reproduction is common, and many other multicellular organism are also capable of reproducing asexually. In animals, asexual reproduction often involves the budding off of a localized mass of cell, which grows by mitosis to form a new individual.
Chapter 2 : Microbial genetics and evolution
Microbiology is the study of single celled creatures or organisms. They are divided in ,Eukaryotes and Prokaryotes
Eukaryotes : Fungi ,Protozoa
Prokaryotes : Bacteria,Archie
Because bacteria are most diverse collection of organisms, it is very hard to say something universally about bacteria. So, this discussion can be inexact concerning all bacteria.
Generally, bacteria are small microorganismes (eg: E coli measures approximately 0,5 in width by 2 μm in length). They have one chromosome, generally circular DNA molecule, but they are very diverse.
Bacteria can be represented schematically:
The center is the nucleoid which is a single chromosome in all bacteria surrounded by cytoplasm, there is an inner membrane and a rigid cell wall.
And some bacteria contain another membrane on the outside (50%) = outer membrane (gram stain is on outer membrane).
Bacteria are divided by fission: for ex E coli is divided every 20’ ⇒1000 (10 generations) in 200′ , 1000000 (20 générations) in 400′ ……….
Bacteria has one DNA molecule and it is typically a circle. And in every case, there is a point on that molecule, where the DNA replication initiates. it is called origin of replication and when it is initiated it progresses in two directions and go around to meet each other at the bottom and at that moment the chromosome is completely replicated.
The speed of DNA polymerase of E coli is 1000 base pairs/sec ,The error rate is 1 Base/generation.
Now, the E coli DNA has 4639221 base pairs ,How long E coli takes to replicate his chromosome? = 70 minutes .It divides par 20 minutes but replicates its DNA per 70 minutes!! How it is possible?? Part of the response is bacteriophage
The DNA is really pressurized in the head of phage ,(Pressure = 6 mega Pascale = 60 atmosphere remember that with a champagne bottle after shaking there is 6 atmospheres).
Phage life style
The second mechanism is shown by lambda phage ,When lambda penetrates, it will see the conditions inside the bacteria; if conditions are bad so it integrates bacterial DNA and stays there until the conditions change.
So, the decision making between giving lytic or lysogenic, is probably the most well understood, of molecular switches in all of biology
In bacteria there is three kinds of exchange of DNA
1) Transformation
What haemophilus will do in order to explore evolutionary space, is it take up DNA from a solution : if that DNA comes from another haemophilus (there are changes in DNA and some are advantageous but some are not).
When it takes DNA from outside it see if it is advantageous or not so integrates or not.
What haemophilus has on it’s surface is a receptor protein that recognizes 11 base pairs sequences of DNA and this is common in haemophilus chromosomes and is uncommon an other chromosomes.
And how it can knows to take a good DNA and not a bad one? Normally, the 11 pairs are different from the phage’ s chromosomes, but sometimes they are the same.The second protection is: restrication-modification system. Each bacteria has an enzyme in it that recognizes a particular sequence of DNA and simply clives that sequence: 4 bases/sequences, 5 bases pairs/sequence, 6 or 8 bases pairs /sequences
CGATCG GCTAGC
When DNA is methylated (CH3 ) the enzyme doEs not cut but otherwise it cut, so if it comes from haemophilus it is methylated, but in viruses and phages it is not.
2) Conjugation
Plasmids is small circular DNA molécules ,F= fertility plasmid .How doses that work?
3) Transduction:
Chapter 3 : cellular metabolism
Cellular metabolism
– Metabolic path ways
– Thermodynamics
– Free energy
– Introduction to enzymes
Overview: the energy of life
The living cell is a miniature chemical factory where thousands of reactions occur.The cell extracts energy and applies energy to perform work. Some organism even convert energy to light as in bioluminescence.
Organization of the chemistry of life into metabolic pathways
A metabolic pathway begins with a specific molecule and ends with a product. Each step is catalyzed by a specific enzyme.Some metabolic pathways break down molecules. Catabolic pathways release energy by breaking down complex molecules into simpler compounds.
Cellular respiration, the breakdown of glucose in the presence of oxygen, is an example of a pathway of catabolism. Some metabolic pathways create molécules. Anabolic pathways consume energy to build complex molecules from simpler ones.The synthesis of protein from amino acids is an example of anabolism.
Bioenergetics is the study of how organisms manage their energy resources .Energy is the capacity to cause change .Energy exists in various forms some of which can perform work:
Kinetic energy is energy associated with motion. Heat (thermal energy) is kinetic energy associated with random movement of atoms or molecules.
Potential energy is energy that mater possesses because of its location or structure. Chemical energy is potential energy available for release in a chemical reaction.Energy can be converted from one form to another.
The laws of energy transformation:
Thermodynamics is the study of energy transformation.A closed system, such as that approximated by liquid in a thermos, is isolated from its surroundings.In an open system, energy and matter can be transformed between the system and its surroundings. Organisms are open systems.
The first law of thermodynamics
According to the first law of thermodynamics, the energy of the universe is constant: Energy can be transferred and transformed, but it cannot be created or destroyed. The first low is also called the principle of conservation of energy.
The second law of thermodynamics
During every energy transfer or transformation, some energy is unusable and is often lost as heat. According to the second law of thermodynamics ,every energy transfer or transformation increases the entropy (disorder) of the universe.
Living cells unavoidably convert organized forms of energy to heat.Spontaneous processes occur without energy input, they can happen quickly or slowly. For a process to occur without energy input, it must increase the entropy of the universe.
Biological order and disorder
Cells create ordered structures from less ordered materials. Organisms also replace ordered forms of matter and energy with less ordered forms. Energy flows into an ecosystem in the form of light and exits in the form of heat.Evolution increases order in organisms locally, but increases disorder in the universe so the second law is not violated.
Equilibrium and metabolism
Reaction in a closed system eventually reach equilibrium and then do no work. Cells are not in equilibrium, they are open system experiencing a constant flow of materials. A defining feature of life is that metabolism is never at equilibrium.A catabolic pathway in a cell releases free energy in a series of reactions. The free energy change of a reaction tells whether or not the reaction occurs spontaneously.
Biologists want to know which reactions occur spontaneously and which requires input of energy.To do so, they need to determine energy changes that occur in chemical reactions.
A living system’s free energy is energy that is available to do work when temperature and pressure are uniform as in a living cell.
Free energy change ( ΔG )
The change in free energy (ΔG) during a process is related to the change in enthalpy or change in total energy (ΔH ), change in entropy (ΔS ) and temperature in Kelvin (T):
ΔG = ΔH – T ΔS
Only process with a negative ΔG are spontaneous. Spontaneous processes can be harnessed to perform work.Free energy stability and equilibrium. Free energy is a measure of a system’s instability its tendency to change to a more stable state. During a spontaneous change, free energy decreases and the stability of a system increases. Equilibrium is a state of maximum stability. A process is spontaneous and can perform work only when it is moving toward equilibrium.
Enzymes
Enzymes (“in yeast”) are the chemical reaction catalysts of biological systems. Most enzymes are proteins and they often use metal ions or prosthetic groups like vitamins to assist catalysis. Many inherited genetic disorders result from a defect or even a total absence of a particular enzyme, or excessive activity of an enzyme. Measurement of enzyme activities in body fluids is important in diagnosing various illnesses.Furthermore many drugs act by altering the activities of enzymes. And enzymes are practical tools in the laboratory.
Enzymes speed up the rates of biochemical reactions. The active site of an enzyme is usually a cleft or pocket where chemistry takes place. A molecule that binds in the active site and is acted upon by the enzyme is called a substrate. As simple equation for an enzyme catalyzed reaction can be written:
E + S ↔ES ↔ EP ↔ E + P
Where E is enzyme, S is substrate , P is product
Enzymes do not alter the reaction equilibrium but they alter the forward and reverse reaction rates. Enzymes remains unchanged after the reaction
Some examples of enzymes catalyzed rate enhancements:
Carbonic anhydrase 10000000 X ,
Thriose phosphate isomerase 1000000000 X ,
Carboxypeptidase A 1000000000000 X
Comparison of catalyzed VS uncatalyzed reaction , the activation energy of the reaction is lower when catalyzed by an enzyme .
One idea about how enzymes work is that they bind to the transition state better than do substrate or product stabilizing the transition state.
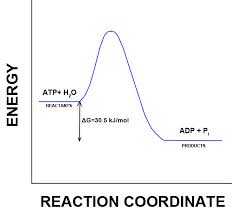
This reaction starts with a substrate having a force energy value and goes to a barrier (calls the transition stats) and go down to form the product and in this reaction the product is at a lower force energy than the reaction this is a spontaneous reaction but may be a very slow reaction in fact many reaction in biology are very slow and we need enzymes to speed up the rates.
We know that enzymes lower the energy of the transition stats and we will see how the enzymes can do that.The equilibrium constant for a reaction is just the ratio of the product over substrate : K = product/substrate
Enzymes speed up metabolic reaction by lowering energy barriers
-A catalyst is a chemical agent that speeds up a reaction without being consumed by reaction.
– An enzyme is a catalytic protein
-Hydrolysis of sucrose by the enzyme Sucrase is an example of an enzyme-catalyzed reaction :
That is a spontaneous reaction in the sense that we could mix up (put) sucrose in to water and wait enough to see the break down to its components. But we must wait really long time (the spontaneous reaction is not a fast reaction). We add enzyme and we can quickly (probably in seconds )see the reaction is speeded up by the enzyme.These enzymes are very specific. for example if we incubate sucrase with other disaccrides like maltose it’s not going to be able to cleave maltose into its components ! and this is another aspect of the enzymes, they are very very specific.
The activation energy barrier
– Every chemical reaction between molecules involves bond breaking and bond forming
-The initial energy needed to start a chemical reaction is called the free energy of activation or activation energy (EA)
-Activation energy is often supplied in the form of heat from the surroundings.
How enzymes lower the EA barrier?
-Enzymes catalyze reactions by lowering the EA barrier.
-Enzymes do not affect the change in free energy (ΔG), (instead they hasten reactions that would occur eventually szpontaneously).
Substrate specificity of enzymes
– The reactant that an enzyme acts on is called the enzyme’s substrate.
– The enzyme binds to its substrate forming an enzyme-substrate complex
-The active site is the region on the enzyme where the substrate binds.
-Induced fit of a substrate brings chemical groups of the active site into positions that enhance their ability to catalyze the reaction.
In general enzymes are really big molecules, their active site is typically small so the enzyme structure is designed to create the architecture that’s necessary to place individual amino acids from the polypeptide segments in the right place in 3D space. In the active site so that substrate combine and chemistry can occur and so we need all that extra protein to create the structure that’s essential for insuring that.
Catalysis in the enzyme’s active site
In an enzymatic reaction, the substraite binds to the active site of the enzyme.
The active site can lower an EA barrier by:
.) orienting substrates correctly
.) Straining substrate bonds.
.) Providing a favorable micro-environment
.) Covalently bonding to the substrate
Binding energy contributes to catalysis in multiple ways:
– Entropy reduction
– Substrate desolvation
– Inducted fit
Exergonic VS. endergonic reactions
-Exergonic reactions release energy to the system. Their ΔG <0 and exergonic reactions proceed spontaneously.
-Endergonic reactions absorb energy from the system. Their ΔG>0 and endergonic reactions are not spontaneous.
ATP Powers Cellular Work by coupling exergonic reactions to endergonic reactions.
To do work cells manage energy resources by energy coupling. The use of an exergonic process to drive an endergonic one.
Most energy coupling in cells is mediated by ATP.
The structure and hydrolysis of ATP (adenosine triphosphate) which is the cell’s energy shuttle.
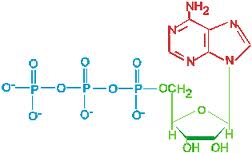
ATP is composed of ribose (a sugar) ,adenine (a nitrogenous base) and three phosphate groups.
The bonds between the phosphate groups of ATP’s tail can be broken by hydrolysis.
Energy is released from ATP when the terminal phosphate bond is broken.
This release of energy comes from the chemical change to a state of lower free energy, not from the phosphate bonds themselves.
How ATP Performs work
The three types of cellular work (mechanical, transport and chemical) are powered by the hydrolysis of ATP.
In the cell, the energy from the exergonic reaction of ATP hydrolysis can be used to drive an endergonic reaction. Overall, the coupled reactions are exergonic.
ATP drives endergonic reactions by phosphorylation, Transferring a phosphate group to some other molecule, such as a reactant.The recipient molecule is now phosphorylated.
The regeneration of ATP
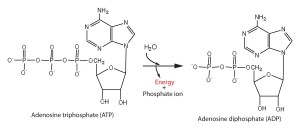
ATP is a renewable resource that is regenerated by addition of a phosphate group to adenosine diphosphate (ADP).
The energy to phosphorylate ADP comes from catabolic reactions in the cell.
The chemical potential energy temporarily stored in ATP drives most cellular work.
Chapter 4: enzyme substrate complex
Substrate specificity of enzymes
-The reaction that an enzyme acts on is called the enzyme’s substrate.
-The enzyme binds to its substrate forming an enzyme-substrate complex
-The active site is the region on the enzyme where the substrate binds.
-Induced fit of a substrate brings chemical groups of the active site into positions that enhance their ability to catalyze the reaction.
in general enzymes are really big molecules their active site is typically small so the enzyme structure is designed to create the architecture that’s necessary to place individual amino acids from the polypeptide segmentes in the right place in 3D space in the active site so that substrates combine and chemistry can occur and so we need all that extra protein to create the structure that’s essential for insuring that the active site is formed correctly and finally induced feet so this is the idea:
Catalysis in the enzyme’s active site
In an enzymatic reaction, the substratet binds to the active site of the enzyme.
The active site can lower the EA barrier:
.) By orienting substrate correctly
.) Straining substrate bonds.
.) Providing a favorable micro environment
.) Covalently bonding to the substrate
Binding energy contributes to catalysis in multiple ways:
– Entropy reduction
– Substrate desolvation
– Inducted fit
Enzyme regulation
Enzyme cofactors
Enzyme inhibitors
Allosteric regulation
1) Cofactors help enzymes to function efficiently
2) Enzymes inhibitors bloc activity through different mechanism :
Competitive, Non competitive
3) Enzyme can be regulated by structural changes
Enzymes can have a really huge effect on reaction rate (the rate enhancement; that’s achieved by an enzyme is more than million X)
Enzymes enhance rates by a very large number .
This is the concept of lock and key.
The lock and key concept for the enzymes is that you have an enzyme that has a binding site for a substrate that is a perfect fit.
And this idea was proposed by Mill Fisher, famous chemist and the idea was that enzymes have incredible specificity for their substrates because they have a binding site that matches that substrate exactly in a chemical sense and this model change chemical reaction so dramatically, and we know that enzyme can have a really huge effect en reaction rates.
This model doses not explain (enzymes that works are structures that stabilize the substrate in the transitional state. If the enzyme binds too well, it is not a good enzyme because it does not help the substrate to approache the transitional state which is a high energy state) .That was the problem with the lock and key model.
In 1950 (nineteen fifties) Dan Coshland proposed a modification to the lock and key model; which he called induced fit.
That means the binding site rather than being an exact match to the structure of the substrates (that simply the lock and key model). It has a shape that is close but not exactly the same as for the substrate. But when the substrate binds there is a structural change that is induced in the enzyme. That actually pushes the substrate closer to the structure. That has in the transitional state.
The difference between lock and key and induced fit is that.
Hexokinase is a beautiful structural example where we see that the substrate coming in and the enzyme closing down around it and inducing a change in the enzyme an substrate binding and then enhances the rate at which the substrate is converted to product.
Cofactors
Cofactors are non-protein enzyme helpers .
Cofactors may be inorganic (such as a metal in ionic form) or organic. An organic cofactor is called coenzyme
Coenzymes include vitamins. Many enzymes have helpers
Cofactors:
1) Tightly bound cofactors are called prosthetic groups
(They are almost like extensions of the protein itself they may not be covalently attached but they are very tightly associated with the enzyme and the enzyme would not be efficient without that group and so that’s what we call prosthetic group)
2) Loosely bound cofactors are called coenzymes
3) An active enzyme lacking the cofactors is called an Apo enzyme the complete enzyme with its cofactor is called a holoenzyme.
Cofactors:
Organic cofactors often share a common chemical feature; can we see it?
Coenzyme A
If you pay attention, you will notice that.
They all have adenine ( adenine ring)
Cofactors:
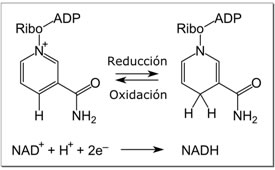
NADH is reversibly oxidized to NAD during electron transport in cells.
Many enzymes contain common structural features that bind NAD/NADH
Regulation of enzyme activity
helps control metabolism
Chemical chaos would result if a cell’s metabolic pathways were not tightly regulated.
A cell does this by switching on or off the genes that encode specific enzymes or by regulating the activity of enzymes
Feedback inhibition
In feedback inhibition, the end product of a metabolic pathway shuts down the pathway.
Feedback inhibition prevents a cell from wasting chemical resources by synthesizing more product than is needed.
Exergonic VS. endergonic reactions
Exergonic reactions release energy to the system and exergonic reactions proceed spontaneously.
Endergonic reactions absorb energy from the system and endergonic reactions are not spontaneous.
ATP Powers Cellular Work by coupling exergonic reactions to endergonic reactions.
A cell does three main kinds of work:
– Chemical
– Transport
– Mechanical
To do work cells manage energy resources by energy coupling. The use of an exergonic process to drive an endergonic one.Most energy coupling in cell is mediated by ATP.
The structure and hydrolysis of ATP
ATP (adenosine triphosphate) is the cell’s energy shuttle.
ATP is composed of ribose (a sugar),adenine (a nitrogenous base) and three phosphate groups.
The bonds between the phosphate groups of ATP’s tail can be broken by hydrolysis.
Energy is released from ATP when the terminal phosphate bond is broken.
This release of energy comes from the chemical change to a state of lower free energy: not from the phosphate bonds themselves.
ATP Hydrolysis
How ATP Performs work
The three types of cellular work (mechanical, transport and chemical) are powered by the hydrolysis of ATP.
In the cell, the energy from the exergonic reaction of ATP hydrolysis can be used to drive an endergonic reaction.Overall, the coupled reactions are exergonic.
ATP drives endergonic reactions by phosphorylation. Transferring a phosphate group to some other molecule, such as a reactant.The recipient molecule is now phosphorylated.
The regeneration of ATP
ATP is a renewable resource that is regenerated by addition of a phosphate group to adenosine diphosphate (ADP).
The energy to phosphorylate ADP comes from catabolic reactions in the cell.
The chemical potential energy temporarily stared in ATP drives most cellular work.
Chapter 5 : bioenergetics
Introduction to bioenergetics
Cellular respiration is composed of three topics:
- Redox reactions (oxidation-reduction)
- Electron transport chain
- Glycolysis
In fact living cells require energy from outside sources. Some animals obtain energy by eating plants and some others feed on other organisms that eat plants, and plants receive energy from sunlight. we can resume, the situation:
Energy flows into an ecosystem as sunlight and leaves as heat.
↓
Photosynthesis generates O2 and organic molecules which are used in cellular respiration.
↓
Cells use chemical energy stored in organic molecules to regenerate ATP, which powers work.
These Catabolic pathways resulting in production of ATP are exergonic (exergonic means energy is released during the process).
Cells do this by different ways.
- Fermentation is a partial degradation of sugars that occurs in the absence of O2 .
- Aerobic respiration is really cellular respiration: it consumes organic molecules and O2 and yields ATP.
- Anaerobic respiration is similar to aerobic respiration but consumes compounds other than O2 .
Redox reactions (oxidation and reduction) involves:
Transfer of electrons during chemical reactions and release energy stored in organic molecules.
The released energy is ultimately used to synthesize ATP.
The principle of redox
Chemical reactions that transfer electrons between reactants are called oxidation-reduction reactions, or redox reactions.
In oxidation, a substance loses electrons, or is oxidized.
In reduction, a substance gains electrons, or is reduced (the same amount of positive charge is reduced)
Example: NaCl → Cl- + Na+
Na= oxidized
Cl = reduced
We can wright it in a general way:
reducing agent + oxidizing agent → reduced agent + oxidized agent
The electron donor is called the reducing agent and the electron receptor is called the oxidizing agent.
Some redox reactions do not transfer electrons but change the electron sharing in covalent bonds.
An example is the reaction between methane and O2
Energy
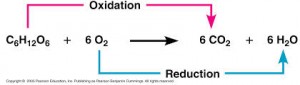
During cellular respiration, the fuel (such as glucose) is oxidized, and O2 is reduced:
Oxygen is a very electro negative agent.
The glucose is the fuel of the organism but the reaction does not happen in one step as shown here and it happens during several steps, this is a very exergonic reaction and the ΔG =-686 Kcal/mol.
The glucose is around and O2 also, why there is no spontaneous combustion? Because the activation energy is high.
stepwise energy harvest via NAD+ and the electron transport chain.
-In cellular respiration, glucose and other organic molecules are broken down in a series of steps.
-Electrons from organic compounds are usually first transferred to NAD+ a coenzyme.
-As an electron acceptor,NAD+ functions as an oxidizing agent during cellular respiration.
-Each NADH (the reduced form of NAD+ ) represents stored energy that can be tapped to synthesize ATP.
CH3OH + NAD+ → NADH + CH2O + H+
-NADH passes the electrons to the electron transport chain.
-Unlike an uncontrolled reaction, the electron transport chain passes electrons in a series of steps instead of one explosive reaction.
-O2 pulls electrons down the chain in an energy-Yielding Tumble
-The energy yielded is used to regenerate ATP.
The stages of cellular respiration: A preview stages
Cellular respiration has three stages:
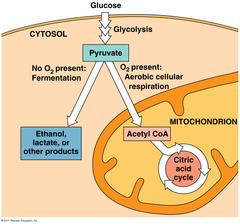
- Glycolysis (breaks down glucose into two molecules of pyruvate)
- The citric acid cycle (completes the breakdown of glucose)
- Oxidative phosphorylation (accounts for most of the ATP synthesis)
Glucose→NADH→electron transport chain→H2O + CO2
Glycolysis is occurring in the cytosol.
And when pyruvate is produced, it enters the citric acid cycle which is going on inside of the mitochondria.
The process that generates most of the ATP is called oxidative phosphorylation because it is powered by redox reactions.
Oxidative phosphorylation, accounts for almost 90% of the ATP generated by cellular respiration.
A smaller amount of ATP is formed in glycolysis and the citric acid cycle by substrate-level phosphorylation.
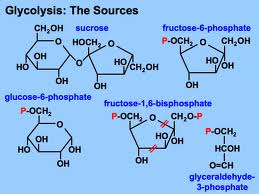
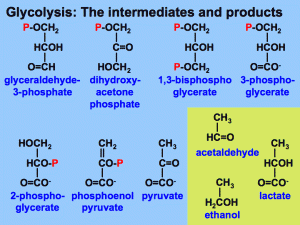
Glycolysis harvests chemical energy, by oxidizing glucose to pyruvate.
Glycolysis (“splitting of sugar”) breaks down glucose, into two molecules of pyruvate.
Glycolysis occurs in the cytoplasm and has two major phases:
- Energy investment phase
- Energy payoff phase
Chapter 6 : cellular energy production
AEROBIC WAY
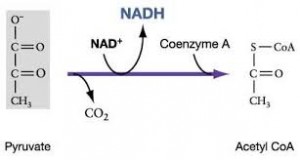
The citric acid cycle completes the energy yielding oxidation of organic molecules. In the presence of O2, pyruvate enters the mitochondrion. Before the citric acid cycle can begin, pyruvate must be converted to acetyl CoA which links the cycle to glycolysis
The first step in this process, is that before the citric acid cycle can actually going, pyruvate has to go across the mitochondrial membrane and just after entering it reacts, with a large enzyme complex, called Pyruvate dehydrogenase (PDH) and that enzyme complex catalysis three steps:
- 1st step: carbon dioxide (CO2) is released
- 2nd step: we have the reduction of NAD+ molecule to NADH
- 3rd step: this reaction is with coenzyme A
Coenzyme A has 3 SH and when it reacts with acetate (pyruvate -Co2),it gives acetyl CoA and it is a high energy bound
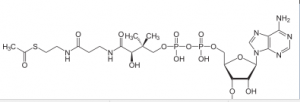
This is a critical step because it allows this molecule to be at the top of an energy hill and goes downhill in the subsequent reaction. pyruvate is a charged molecule so it cannot freely cross the mitochondrial membrane and in fact it must be transported across the membrane by an active transport process using a transport protein!!! CoA is derived from a B vitamin (Thiamine) thiamine deficiency causes Beriberi
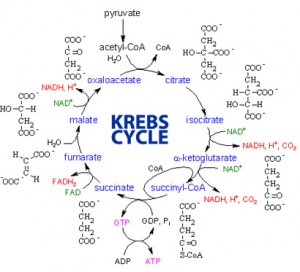
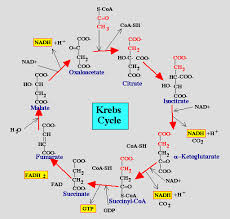
The citric acid cycle also called the Krebs cycle or the tricarboxylic acid (TCA) cycle, takes place within the mitochondrial matrix
The cycle oxidizes organic fuel derived form pyruvate generating 1 ATP, 3 NADH, 1 FADH2 per turn
The mitochondria are the major site of ATP production in the dark but the chloroplast is the site of ATP production in the light.In the bacteria the actual enzymes that carry out this cycle are in the cytosol because they don’t have mitochondria and the plasma membrane of bacteria contains the enzymes and complexes that carry out oxidative phosphorylation.
The citric acid cycle
- The citric acid cycle has eight steps each catalyzed by a specific enzyme.
- The acetyl group of acetyl CoA joins the cycle by combining with oxaloacetate forming citrate.
- The next seven steps decompose the citrate back to oxaloacetate making the process a cycle.
The NADH and FADH2 produced by the cycle relay electrons extracted from food to the electron transport chain.
Steps of Krebs cycle:
During oxidative phosphorylation chemiosmosis couples electron transport to ATP synthesis.
- Following glycolysis and the citric acid cycle, NADH and FADH2 account for most of the energy extracted from food
- These two electron carriers donate electrons to the electron transport chain which power ATP synthesis via oxidative phosphorylation.
The pathway of electron transport
- The electron transport chain is in the cristae of the mitochondrion
- Most of the chain’s components are proteins, which exist in multiprotein complexes
- The carriers, alternate reduced and oxidized states, as they accept and donate electrons.
- Electrons drop in free energy as they go down the chain and are finally passed to O2 forming H2O
Electrons are transferred from NADH or FADH2 to the electron transport chain
Electrons are passed through a number of proteins including cytochromes (each with an iron atom) to O2.
The electron transport chain generate no ATP. The chain’s function is to break the large free–energy drop, from food to O2, into smaller steps that release energy in manageable amounts
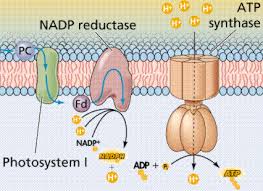
Chemiosmosis: the energy-coupling mechanism
Electron transfer in the electron transport chain causes proteins to pump H+ from the mitochondrial matrix to the intermembrane space.H+ then moves back across the membrane passing through channels in ATP synthase.ATP synthase uses the exergonic flow of H+ to drive phosphorylation of ATP.This is an example of chemiosmosis the use of energy in H+ gradient to drive cellular work.The energy stored in a H+ gradient across a membrane couples the redox reactions of the electron transport chain to ATP synthesis.The H+ gradient is referred to as a proton – motive force, emphasizing its capacity to do work.
About40% of the energy in a glucose molecule is transferred to ATP during
cellular respiration,making about 38 ATP molecules.
Glucose oxidation ΔG = -686 kcal/mole.
ADP +Pi →ATP ΔG = 7,3 kcal/mol ⇒ 7,3x 38 = 277 kcal this make roughly
40 % of the total energy of Glucose.
Fermentation and anaerobic respiration enable cells to produce ATPwithout the use of oxygen
- Most cellular respiration requires O2 to produce ATP.
- Glycolysis can produce ATP with or without O2 (in aerobic or anaerobic conditions)
- In the absence of O2, glycolysis couples with fermentation or anaerobic respiration to produce ATP.
- Anaerobic respiration uses an electron transport chain with an electro – acceptor other than O2, for example sulfate.
- Fermentation uses phosphorylation instead of an electron transport chain to generate ATP.
Types of fermentation
- Fermentation consists of glycolysis plus reactions that generates NAD+ which can be reused by glycolysis.
- Two common types are alcohol fermentation and lactic acid fermentation.
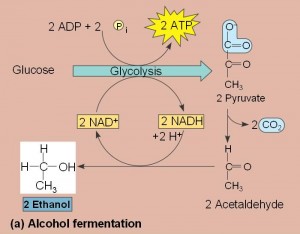
- In alcohol fermentation, pyruvate is converted to ethanol in two steps with the first releasing CO2.
- Alcohol fermentation by yeast is used in brewing, wine making and baking.
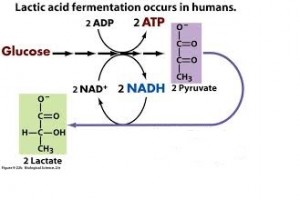
- In lactic acid fermentation pyruvate is reduced by NADH forming lactate as an end product with no release of CO2.
- Lactic acid fermentation by some fungi and bacteria is used to make cheese and yogurt.
- Human muscle cells use lactic acid fermentation to generate ATP when O2 is scarce.
Lactate is recycled to pyruvate in the liver (cori cycle) so in this pathway, we have 2 ATP produced. This is a much less efficient pathway.
Fermentation and aerobic respiration (compared)
- Both processes, use glycolysis to oxidize glucose and other organic fuels to pyruvate.
- The processes have different final electron acceptors, an organic molecule (such as pyruvate or acetaldehyde) in fermentation and O2 in cellular respiration.
- Cellular respiration produces 38 ATP per glucose molecule, fermentation 2 ATP per glucose molecule.
- Obligate anaerobes, carry out, fermentation or anaerobic respiration and can not survive in the presence of O2.
- Yeast and many bacteria are facultative anaerobes, meaning that they can survive using either fermentation or cellular respiration.
- In a facultative anaerobe pyruvate is a fork in the metabolic road that leads to two alternative catabolic routes.Human muscles cells are facultative anaerobs.
The evolutionary significance of Glycolysis
- Glycolysis occurs in nearly all organisms.
- Glycolysis probably, evolved in ancient prokaryotes before there was oxygen in the atmosphere.
- Glycolysis and the citric AC cycle, connect to many other metabolic pathways.
- Glycolysis and the citric AC cycle, are major intersections to various catabolic and anabolic pathways.
The versatility of catabolism
- Catabolic pathways, funnel electrons from many kinds of organic molecules into cellular respiration.
- Glycolysis accepts a wide range of carbohydrates.
- Proteins must be digested to amino acids, amino acids can feed glycolysis or the citric acid cycle.
- Fats are digested to glycerol (used in glycolysis) and fatty acids (used in generating acetyl COA).
- Fatty acids are broken down by beta oxidation and yield acetyl COA.
- An oxidized gram of fat produces more than twice as much ATP as an oxidized gram of carbohydrate.
Biosynthesis (anabolic pathway)
- The body uses small molecules to build other substances.
- These small molecules may come directly from food, from glycolysis or from the citric acid cycle.
Regulation of cellular respiration via feedback mechanisms
- Feedback inhibition is the most common mechanism for control.
- If ATP concentration begins to drop, respiration speeds up, when there is plenty of ATP, respiration slows down.
- Control of catabolism is based mainly on regulating the activity of enzymes at strategic points in the catabolic pathway.
Chapter 7 : Photosynthesis
Photosynthesis
Photosynthesis is the process that converts solar energy to chemical energy. Directly or indirectly, photosynthesis nourishes almost the entire living worlds
-Autotrophs sustain themselves without eating anything derived from other organisms
-Autotrophs are the producers of the biosphere, producing organic molecules from CO2 and other inorganic molecules.
-Almost all plants are photoautotrophs, using the energy of sunlight to make organic molecules from H2O and CO2.
-Heterotrophs obtain their organic material from other organisms.
-Heterotrophs are the consumers of the biosphere.
-Almost all heterotrophs, including humans, depend on photoautotrophs for food and O2
Photosynthesis
-Photosynthesis converts light energy to the chemical energy of food.
-Chloroplasts are structurally similar to and likely evolved from photosynthetic bacteria.
-The structural organization of these cells allows for the chemical reactions of photosynthesis.
-The chloroplasts have their own DNA and their own ribosomes.
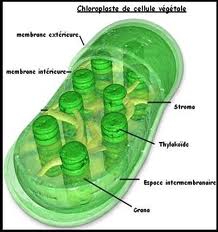
Chloroplasts: the site of photosynthesis in plants
-Leaves are the major locations of photosynthesis
-Their green color is from chlorophyll, the green pigment within chloroplasts
-Light energy absorbed by chlorophyll drives the synthesis of organic molecules in the chloroplast
-CO2 enters and O2 exits the leaf through microscopic pores called stomata
-Chloroplasts are found mainly in cells of the mesophyll, the interior tissue of the leaf
-A typical mesophyll cell has 30-40 chloroplasts.
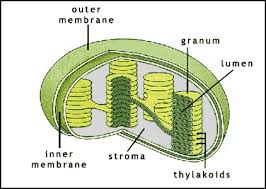
-The chlorophyll is in the membranes of thylakoids (connected sacs in the chloroplast),thylakoids may be stacked in columns called Grana.
-Chloroplasts also contain stroma a dense fluid
Tracking atoms through photosynthesis
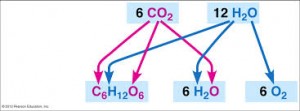
– Photosynthesis can be summarized as the following equation:
6CO2 + 12H2O + light energy → C6H12O6 + 6O2 + 6H2O
– Chloroplasts, split H2O into hydrogen and oxygen, incorporating the electrons of hydrogen into sugar molecules
Photosynthesis is a redox process in which H2O is oxidized and CO2 is reduced
CO2 + H2O→⌊ch2o⌋+O2
CO2 + H2S →⌊CH2O⌋+2S
The two stages of photosynthesis
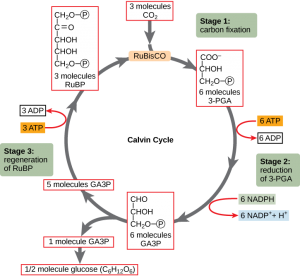
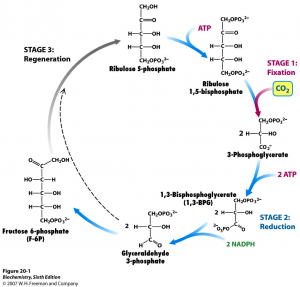
Photosynthesis consists of the light reactions (the photo part) and Calvin cycle (the synthesis part).
The light reactions (in the thylakoids)
- Split H2O
- Release O2
- Reduce NADP+ to NADPH
- Generate ATP from ADP by photo phosphorylation.
The Calvin cycle (in the stroma) forms sugar from CO2 using ATP and NADPH.
- The Calvin cycle begins with carbon fixation, incorporating CO2 into organic molecules.
- Chloroplasts are solar–powered chemical factories.
- Their thylakoids transform light energy into the chemical energy of ATP and NADPH
The nature of sunlight
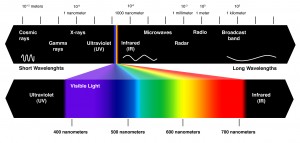
– Light is a form of electromagnetic energy, also called electromagnetic radiation
– Like other electromagnetic energy light travels in rhythmic waves
– Wavelength is the distance between crests of waves
– Wavelength determines the type of electromagnetic energy
– the electromagnetic spectrum is the entire range of electromagnetic energy, or radiation.
– Visible light consists of wavelengths (including those that drive photosynthesis) that produce colors we can see.
– Light also behaves as though it consists of discrete particles called photons.
Photosynthetic pigments
Pigments are substances that absorb visible light
Different pigments absorb different wavelengths
Wavelengths that are not absorbed are reflected or transmitted
Leaves appear green because chlorophyll reflects and transmits green light
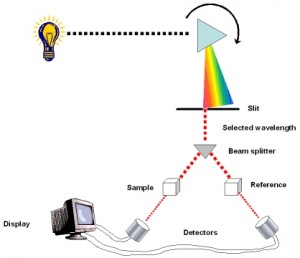
A spectrophotometer measures a pigment’s ability to absorb various wavelengths. This machine sends light through pigments and measures the fraction of light transmitted at each wavelength
spectrophotometer
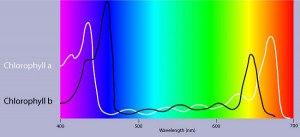
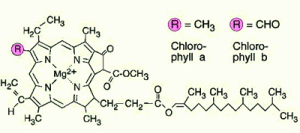
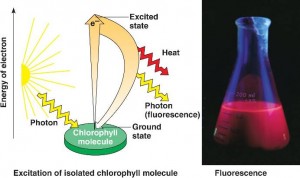
An absorption spectrum is a graph plotting a pigment light absorption versus wavelength .The absorption spectrum of chlorophyll a suggests, that violet-blue and red light, work best, for photosynthesis . An action spectrum, profiles the relative effectiveness of different wavelengths of radiation in driving a process. The action spectrum of photosynthesis was first demonstrated in 1883 by Theodor W.Engelmann. In his experiment he exposed different segments of a filamentous algae to different wavelengths.Areas receiving wavelengths favorable to photosynthesis produced excess O2. He used the wavelength of aerobic bacteria clustered along the algae as a measure of O2 production.Chlorophyll a is the main photosynthesis pigment.Accessory pigments such as chlorophyll b broaden the spectrum used for photosynthesis. Accessory pigments called carotenoids absorb excessive light that would damage chlorophyll b
Structure of pigments
Excitation of chlorophyll by light
When a pigment absorbs light, it goes from a ground state to an excited state, which is unstable. When excited electrons fall back to the ground state, photons are given off, an afterglow called fluorescence. If illuminated an isolated solution of chlorophyll will fluoresce, giving off light and heat.
Chapter 8 : TRANSCRIPTION
. The central dogma of molecular biology
The central dogma is the classic sequence of events :
DNA produces RNA by transcription and RNA produces proteins ( structural proteins and Enzymes ) by translation .
Transcription: making of RNA (taking the information of DNA and copying the information to (transitional form) RNA which is unstable.
Translation: is the act of synthesizing a protein from the information in the RNA.
Against central dogma:
-Enzymes can be also certain forms of RNA.
-Now we can take RNA and make DNA from that.
-There are enzymes that can make RNA from RNA.
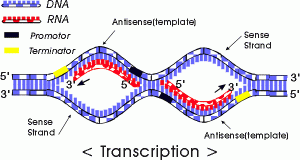
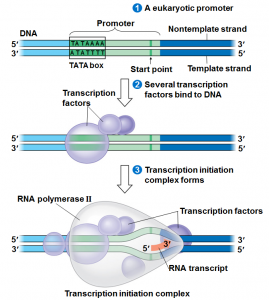
We will begin by transcription in prokaryotes.Typically in prokaryotes the transcription produces an RNA that encodes for more than one protein. Transcription starts at a promoter and make RNA at the same direction that DNA polymerase do for DNA (5’→3’) direction. RNA polymerase which recognizes promoter and does transcription (promoter is defined the site that bound by RNA polymerase and where transcription starts).
There are 2 parts to a promoter : there are so called -10 sequences and so called -35 sequences. What the -10 and -35 refer to is the distance in base pairs from the first base of DNA that is copied into RNA.These sequences are highly characteristic of all genes in the case of -10 of the classes and the genes on the -35 sequences.
The -35 sequence is recognized by a protein (a family of proteins referred to) as sigma factors, these are regulatory proteins. Bacterial RNA polymerases are formed by 4 different subunits (2 copies of big subunits called α and copies of β and β’) ⇒ α2ββ’.
α2ββ’ is the holoenzyme and then σ factor is a regulatory subunit and comes in many varieties. There are many σ factors ,the key thing with σ is that they are specific to particular promoters ,examples :
1)Heatshok
2) sporulation
There are many factors, the key thing with factors is that they are specific to particular promoters.
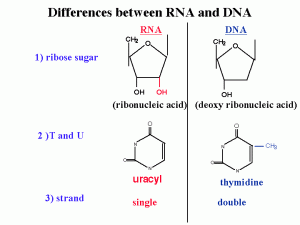
Differences between DNA and RNA :
1)2’ OH is special for RNA
DNA is deoxy. This OH group makes RNA unstable and vulnerable by enzymes or bases or different kinds of biophysical conditions. So RNA is designed to be unstable. Why RNA must be unstable (the earliest forms of life used RNA as a genetic material). Why we use an unstable molecule? probably it’s for the control of gene expression.
2) uracyl instead of thymidine
3) no primer required for the synthesis of RNA from RNA
4) synthesized in 5’→3’ direction (the chemistry involved is identical to DNA polymerase) :Initiation-elongation-termination
Step 1 :closed promoter complex
Step 2: formation of open promoter complex which is 17 bases pairs (normally there are 10 bases pairs by turn of helix) So 17 is almost 2 turns of helix in this situation, RNA polymerase start to synthesize RNA.
How we can confirm that? by DNA foot printing….







![image082[1]](http://borzuya.com/wp-content/uploads/2014/05/image0821-300x270.jpg)