Cellular metabolism
– Metabolic path ways
– Thermodynamics
– Free energy
– Introduction to enzymes
Overview: the energy of life
The living cell is a miniature chemical factory where thousands of reactions occur.The cell extracts energy and applies energy to perform work. Some organism even convert energy to light as in bioluminescence.
Organization of the chemistry of life into metabolic pathways
A metabolic pathway begins with a specific molecule and ends with a product. Each step is catalyzed by a specific enzyme.Some metabolic pathways break down molecules. Catabolic pathways release energy by breaking down complex molecules into simpler compounds.
Cellular respiration, the breakdown of glucose in the presence of oxygen, is an example of a pathway of catabolism. Some metabolic pathways create molécules. Anabolic pathways consume energy to build complex molecules from simpler ones.The synthesis of protein from amino acids is an example of anabolism.
Bioenergetics is the study of how organisms manage their energy resources .Energy is the capacity to cause change .Energy exists in various forms some of which can perform work:
Kinetic energy is energy associated with motion. Heat (thermal energy) is kinetic energy associated with random movement of atoms or molecules.
Potential energy is energy that mater possesses because of its location or structure. Chemical energy is potential energy available for release in a chemical reaction.Energy can be converted from one form to another.
The laws of energy transformation:
Thermodynamics is the study of energy transformation.A closed system, such as that approximated by liquid in a thermos, is isolated from its surroundings.In an open system, energy and matter can be transformed between the system and its surroundings. Organisms are open systems.
The first law of thermodynamics
According to the first law of thermodynamics, the energy of the universe is constant: Energy can be transferred and transformed, but it cannot be created or destroyed. The first low is also called the principle of conservation of energy.
The second law of thermodynamics
During every energy transfer or transformation, some energy is unusable and is often lost as heat. According to the second law of thermodynamics ,every energy transfer or transformation increases the entropy (disorder) of the universe.
Living cells unavoidably convert organized forms of energy to heat.Spontaneous processes occur without energy input, they can happen quickly or slowly. For a process to occur without energy input, it must increase the entropy of the universe.
Biological order and disorder
Cells create ordered structures from less ordered materials. Organisms also replace ordered forms of matter and energy with less ordered forms. Energy flows into an ecosystem in the form of light and exits in the form of heat.Evolution increases order in organisms locally, but increases disorder in the universe so the second law is not violated.
Equilibrium and metabolism
Reaction in a closed system eventually reach equilibrium and then do no work. Cells are not in equilibrium, they are open system experiencing a constant flow of materials. A defining feature of life is that metabolism is never at equilibrium.A catabolic pathway in a cell releases free energy in a series of reactions. The free energy change of a reaction tells whether or not the reaction occurs spontaneously.
Biologists want to know which reactions occur spontaneously and which requires input of energy.To do so, they need to determine energy changes that occur in chemical reactions.
A living system’s free energy is energy that is available to do work when temperature and pressure are uniform as in a living cell.
Free energy change ( ΔG )
The change in free energy (ΔG) during a process is related to the change in enthalpy or change in total energy (ΔH ), change in entropy (ΔS ) and temperature in Kelvin (T):
ΔG = ΔH – T ΔS
Only process with a negative ΔG are spontaneous. Spontaneous processes can be harnessed to perform work.Free energy stability and equilibrium. Free energy is a measure of a system’s instability its tendency to change to a more stable state. During a spontaneous change, free energy decreases and the stability of a system increases. Equilibrium is a state of maximum stability. A process is spontaneous and can perform work only when it is moving toward equilibrium.
Enzymes
Enzymes (“in yeast”) are the chemical reaction catalysts of biological systems. Most enzymes are proteins and they often use metal ions or prosthetic groups like vitamins to assist catalysis. Many inherited genetic disorders result from a defect or even a total absence of a particular enzyme, or excessive activity of an enzyme. Measurement of enzyme activities in body fluids is important in diagnosing various illnesses.Furthermore many drugs act by altering the activities of enzymes. And enzymes are practical tools in the laboratory.
Enzymes speed up the rates of biochemical reactions. The active site of an enzyme is usually a cleft or pocket where chemistry takes place. A molecule that binds in the active site and is acted upon by the enzyme is called a substrate. As simple equation for an enzyme catalyzed reaction can be written:
E + S ↔ES ↔ EP ↔ E + P
Where E is enzyme, S is substrate , P is product
Enzymes do not alter the reaction equilibrium but they alter the forward and reverse reaction rates. Enzymes remains unchanged after the reaction
Some examples of enzymes catalyzed rate enhancements:
Carbonic anhydrase 10000000 X ,
Thriose phosphate isomerase 1000000000 X ,
Carboxypeptidase A 1000000000000 X
Comparison of catalyzed VS uncatalyzed reaction , the activation energy of the reaction is lower when catalyzed by an enzyme .
One idea about how enzymes work is that they bind to the transition state better than do substrate or product stabilizing the transition state.
This reaction starts with a substrate having a force energy value and goes to a barrier (calls the transition stats) and go down to form the product and in this reaction the product is at a lower force energy than the reaction this is a spontaneous reaction but may be a very slow reaction in fact many reaction in biology are very slow and we need enzymes to speed up the rates.
We know that enzymes lower the energy of the transition stats and we will see how the enzymes can do that.The equilibrium constant for a reaction is just the ratio of the product over substrate : K = product/substrate
Enzymes speed up metabolic reaction by lowering energy barriers
-A catalyst is a chemical agent that speeds up a reaction without being consumed by reaction.
– An enzyme is a catalytic protein
-Hydrolysis of sucrose by the enzyme Sucrase is an example of an enzyme-catalyzed reaction :
That is a spontaneous reaction in the sense that we could mix up (put) sucrose in to water and wait enough to see the break down to its components. But we must wait really long time (the spontaneous reaction is not a fast reaction). We add enzyme and we can quickly (probably in seconds )see the reaction is speeded up by the enzyme.These enzymes are very specific. for example if we incubate sucrase with other disaccrides like maltose it’s not going to be able to cleave maltose into its components ! and this is another aspect of the enzymes, they are very very specific.
The activation energy barrier
– Every chemical reaction between molecules involves bond breaking and bond forming
-The initial energy needed to start a chemical reaction is called the free energy of activation or activation energy (EA)
-Activation energy is often supplied in the form of heat from the surroundings.
How enzymes lower the EA barrier?
-Enzymes catalyze reactions by lowering the EA barrier.
-Enzymes do not affect the change in free energy (ΔG), (instead they hasten reactions that would occur eventually szpontaneously).
Substrate specificity of enzymes
– The reactant that an enzyme acts on is called the enzyme’s substrate.
– The enzyme binds to its substrate forming an enzyme-substrate complex
-The active site is the region on the enzyme where the substrate binds.
-Induced fit of a substrate brings chemical groups of the active site into positions that enhance their ability to catalyze the reaction.
In general enzymes are really big molecules, their active site is typically small so the enzyme structure is designed to create the architecture that’s necessary to place individual amino acids from the polypeptide segments in the right place in 3D space. In the active site so that substrate combine and chemistry can occur and so we need all that extra protein to create the structure that’s essential for insuring that.
Catalysis in the enzyme’s active site
In an enzymatic reaction, the substraite binds to the active site of the enzyme.
The active site can lower an EA barrier by:
.) orienting substrates correctly
.) Straining substrate bonds.
.) Providing a favorable micro-environment
.) Covalently bonding to the substrate
Binding energy contributes to catalysis in multiple ways:
– Entropy reduction
– Substrate desolvation
– Inducted fit
Exergonic VS. endergonic reactions
-Exergonic reactions release energy to the system. Their ΔG <0 and exergonic reactions proceed spontaneously.
-Endergonic reactions absorb energy from the system. Their ΔG>0 and endergonic reactions are not spontaneous.
ATP Powers Cellular Work by coupling exergonic reactions to endergonic reactions.
To do work cells manage energy resources by energy coupling. The use of an exergonic process to drive an endergonic one.
Most energy coupling in cells is mediated by ATP.
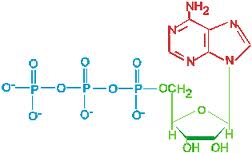
The structure and hydrolysis of ATP (adenosine triphosphate) which is the cell’s energy shuttle.
ATP is composed of ribose (a sugar) ,adenine (a nitrogenous base) and three phosphate groups.
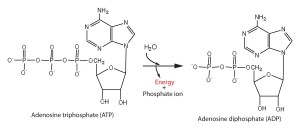
The bonds between the phosphate groups of ATP’s tail can be broken by hydrolysis.
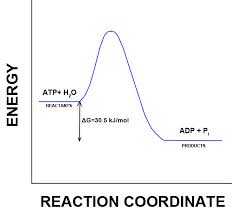
Energy is released from ATP when the terminal phosphate bond is broken.
This release of energy comes from the chemical change to a state of lower free energy, not from the phosphate bonds themselves.
How ATP Performs work
The three types of cellular work (mechanical, transport and chemical) are powered by the hydrolysis of ATP.
In the cell, the energy from the exergonic reaction of ATP hydrolysis can be used to drive an endergonic reaction. Overall, the coupled reactions are exergonic.
ATP drives endergonic reactions by phosphorylation, Transferring a phosphate group to some other molecule, such as a reactant.The recipient molecule is now phosphorylated.
The regeneration of ATP
ATP is a renewable resource that is regenerated by addition of a phosphate group to adenosine diphosphate (ADP).
The energy to phosphorylate ADP comes from catabolic reactions in the cell.
The chemical potential energy temporarily stored in ATP drives most cellular work.