Organic chemistry is the chemistry of carbon and its compounds. Carbon is one element of the Mendeleev table among many others, so why is there a complete section of chemistry related to this particular element? Carbon has a valence of 4 and can thus bind with up to 4 other elements of the periodic table. To that point there is nothing extraordinary. However, where in the inorganic chemistry atoms can only bind together to form small molecules, carbon based molecules can form long and stable chains with a rich variety of functional groups.
Organic chemistry is so called because carbon is the essential constituent of living species: proteins, ADN, lipids, sugars or fats are a few example of organic compounds, made of a structure of carbon wearing functional groups allowing them to interact together, becoming more than just their simple addition but to form a functionning macrosystem where each molecule has a specific role to sustain a stable living body.
Organic chemistry is thus very important in the science of the living, but is not limited to that. Plastic is an organic compound that we find everywhere, oil, toothpaste, shampoos, clothes, deodorants, etc. are products of organic chemistry.
To adventure ourselves in this vast world that is organic chemistry, we will first discuss over alkanes, their structures and how scientists call them, and how they are represented. Later we will introduce the different functional groups that we can find on organic compounds and finally how organic compounds react, how we can produce them or modify them.
Alkanes
Alkanes are compounds only composed of carbon and hydrogen atoms. Hydrogen has a valence of 1, meaning that it can only make one liaison with another atom. A single carbon will thus bind with 4 hydrogen to form a neutral species CH4. This molecule is called methane and is a gas in normal conditions. The liaisons are covalent liaisons. The carbon is slightly more electronegative than the hydrogen but it is not important at this moment of the lesson. Just remember that CH4 will not dissociate from a proton and is not an acid.
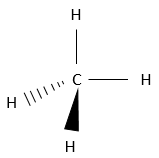
There are several ways to represent this molecule. The fully developed representation is as follows:
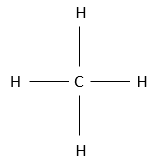
In this representation of the methane, all the liaisons are shown by a full line connecting the atoms. All the atoms are shown independently as well. This representation is in two dimensions and the hydrogen atoms in 3D are in reality not on a single plane. The structure of lowest energy is the structure where the hydrogen’s are the most separated from each other. Indeed, hydrogen’ss have a given volume and are repelling each other. To obtain that structure, an angle of 109.5° separates the liaisons. It leads to a tetrahedric structure.
Most of the time, there is no point in showing the complicated structure of large molecules. It would only make it harder to see the important information of the molecule. However, it is sometime important to know in which direction goes one particular liaison. In this case, the lines representing the liaisons take different forms depending on their orientation. In the plane of the page, liaisons are still represented by a simple line. Two other cases are possible: Liaisons can go in the direction of the reader or in the opposite direction. Liaisons going toward the reader are represented by a black triangle one peak of which is connected to the atom on the plane of the page and the two other points are connected to the atom out of the plane. This way, the triangle looks like a line going wider from the atom in the plane to the atom closer to the reader.
For liaisons going in the opposite direction, atoms are connected by traits (parallel or perpendicular to the liaison direction your choice). For methane, it gives the following 3D representation:
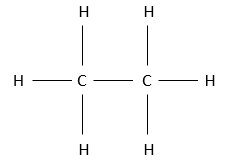
The alkane possessing a structure of 2 carbon atoms is the ethane. It is a gas as the methane. To form the “bone” structure of the ethane, the two carbon atoms bind together through a covalent liaison. Obviously they have the same electronegativity. From their 4 electrons, one is thus used by each carbon to bind with the other one. 6 hydrogen will thus complete the structure. As in inorganic chemistry, the octet rule is respected: to be stable, a carbon has to have 8 electrons (an octet) around it: its 4 electrons and 4 electrons of the other atoms with which it shares a bond. Each carbon of the ethane is thus bound with a carbon and with 3 hydrogens. It is the single possible structure for such compound. In no way one carbon would wear 5 hydrogens and the other carbon 1 or more.
The fully represented structure of the ethane is thus
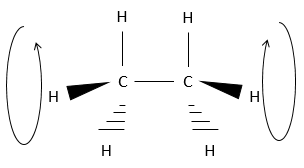
If we want to represent it in 3D, it would be:
However, it is to be said that atoms can rotate in the axe of a simple liaison. The 6 hydrogen are thus turning in circle around the axis made by the two carbons, as shown above. The Hydrogen’s are rotating almost freely around this axis.
Each hydrogen has a given volume and feels the atoms in its vicinity (steric hindrance). When rotating, the distance between hydrogen’s on a same carbon is constant. However, the distance with the closest hydrogen carried by the other carbon changes.
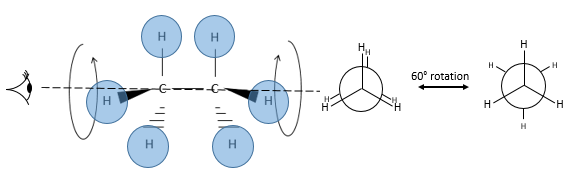
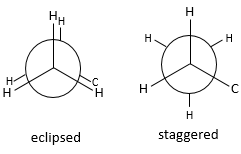
The relative positions of substituents can be showed through the Newman projection. The molecule is observed along its C-C axis. The first C (proximal carbon) is represented by a circle from the centre of which three lines are going out. These lines are the liaisons of this carbon. The second carbon (distal carbon) is hidden by the first one but one part of the liaison is visible.
Fixing the hydrogen’s of the proximal carbon, only the hydrogen’s of the distal carbon can move. Two cases can be observed:
- The hydrogen’s of the distal and of the proximal carbons are on the same spots, or so called eclipsed
- The hydrogen’s are not on the same spots, or so called staggered
A maximum of energy is reached in the eclipse conformation because repulsion between the hydrogen’s is maximal in this conformation. A rotation of 60° from this conformation leads to a minimum of energy, the hydrogen’s being as far away from each other as they can be. A molecule that has to maintain hydrogen’s (or substituents) eclipsed has a higher energy than a molecule of same composition with staggered hydrogen’s. The difference in energy here is not very important and the rotation is effective. During the rotation, the molecule passes more time in the staggered conformation (smaller energy). If substituents were on the molecule, the steric hindrance increases with the radius of the substituent. In some cases, the rotation can be blocked by the presence of voluminous substituents.
The semi representation of the ethane is
In this representation, the carbons are regrouped with the atoms they are wearing but not sharing. The liaisons between C and H are thus not showed in this representation. If a Hydrogen atom was replaced by a chlorine atom, for example, the half representation would be:
Adding a third carbon atom to the chain, C3H8, the propane, is still a linear alkane. The structure in triangle where each carbon binds with two other carbon exists but is not very stable. You maybe have spotted that the formula of linear alkanes has a general model: CnH2n+2. For each atom of carbon added to the first one, 2 hydrogen are to be added.
There are two ways now to add a fourth carbon to obtain a butane molecule. The chain can be extended by its extremities or by its middle. When the chain is linear, we add n- before the name of the compound. n-butane is thus
![]()
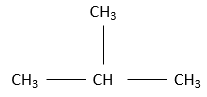
If the chain is extended by its middle, we name this compound isobutane
The iso prefix is used only for a few compound wherein a carbon wear two terminal CH3. n-butane and isobutane share the same formula C4H10 but don’t have the same structure. Such kind of compound is called an isomer of constitution. The greater the number of carbon in an alkane, the greater the number of isomers.
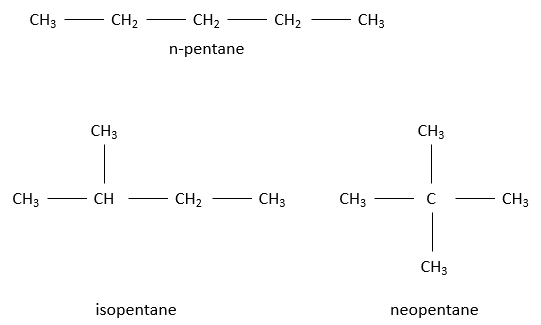
For a 5 carbon chain, the fifth carbon can be added at one extremity of the n-butane to obtain n-pentane, equivalently at any extremity of the isobutane (it is the same as adding the C on one CH2 of the n-butane) to obtain isopentane, or on the CH to obtain neopentane.
Names of the alkanes:
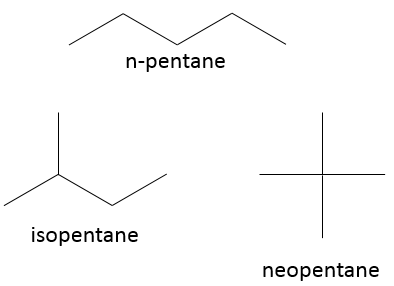
Another representation of organic molecules is the skeleton representation. In this representation, carbons and hydrogens are not shown. The liaisons between carbons are still shown as full lines and are connected together with an angle at the position of the carbon atoms. Without angle, we could not differentiate a chain of 6 carbons from a chain of 7. Generally, the angle is approximately 120° so that if a carbon is bound to three other species (other than H), each liaison is equally distant. If a carbon is bound to 4 species, the angle is 90°.
For example, the pentanes showed above are represented
This bone structure is the representation that is usually used. Only important informations are shown. The number of hydrogen on the different carbons of the bone are determined by the number of liaisons that the carbon has. It is thus pointless to show it. Moreover, this representation is faster to write and takes less space.
There is a given method to name organic compounds. An alkane as a functional group has the same name except that the -ane is replaced by -yl. For example, isobutane is also called methylpropane because a methyl is fixed at a linear chain of 3 carbons, i.e. a propane chain.
C4H9Cl is a Chlorobutane. With this name, we know the components of the compound but not its complete structure. The connectivity in the butane and between the butane and the chlorine are not known.
The rules to name a compound are
- the longest chain is the main one. However if a functional group is on one chain, the main chain has to wear it.
- tag a number to each of the carbon from one side of the main chain to the other. The carbon wearing a functional group which is the closest of an extremity has to have the smallest number.
- Next, we name the compound by writing first the groups out of the main chain, with their number as prefix, in the alphabetical order, followed by the main chain with its group.
- our examples are thus named
- If several identical functional groups are on different carbons, the prefix are separated by a , and their number is indicated by bi, tri,…
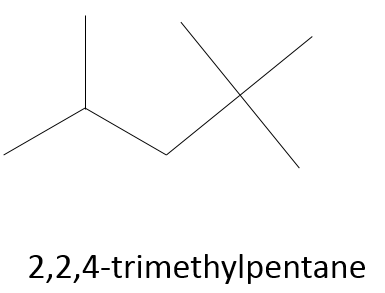
- ex: the isooctane is the 2,2,4-trimethylpentane, meaning that a total of 3 methyl groups are on a main chain of 5 carbons. Two methyls are on the carbon #2 and one on the #4. It is 2,2,4-trimethylpentane and not 2,4,4-trimethylpentane because we favor the carbon wearing more groups.
Halogenoalkanes
Simply said, halogenoalkanes are alkanes wearing one or more halogen. It is simply said but halogenoalcanes are not so easily made. They are made from a dihalogen and an alkane through a radical reaction during which a proton has to be removed from the alkane. This step of the reaction is not favorable but can be made through heavy heating (300° for the chloromethane). Also, the position of the halogen is not completely fixed. The proton removed during the reaction is easier removed from a carbon in the chain than at an extremity but the high temperature makes both positions possible (the distribution depends on the temperature)
Halogens have a higher electronegativity than C and they generate a dipole from C to X. A carbon wearing an halogen is thus poor in electrons and will consequently be targeted by reactants rich in electrons. The reactivity of halogenoalkanes will be seen in a further section.
Chiral molecules
We have already see that for one given formula, several different molecules may exist. When the connectivity differs, these are isomers of constitution.
Ex: butane and methylpropane, ethanol and methoxymethane.
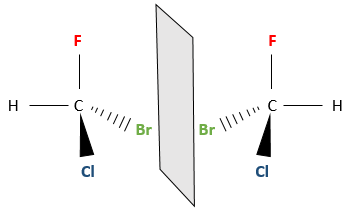
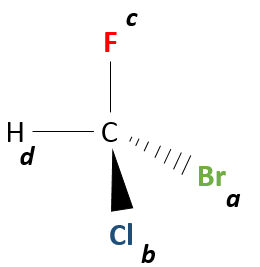
Even on a single carbon, the connectivity may change. Stereoisomers are isomers of same connectivity but with different spatial positioning. The bromochlorofluoromethane has two stereoisomers forms.
These two molecules are mirror images one of the other. It is said that they are chiral if the molecule and its mirror image do not superimpose. These particular stereoisomers are called enantiomers.
A good way to explain chirality is to look at our hands. The left hand is the mirror image of the right hand (and vice versa). However, we cannot superimpose them.
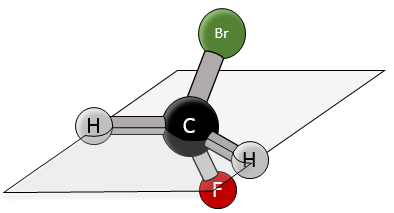
Chirality is related to the carbon that wears several different groups. It is a stereocentre. Stereocentres are often indicated by an asterisc. If a plane of symetry exists for the molecule, this molecule is achiral (><chiral) and this molecule and its mirror image can superimpose somehow. For example, Bromofluoromethan is achiral because a plane of symetry can be drawn, passing by the two hydrogen atoms.
Our body is able to distinguish enantiomers from each other. For some medicines, one enantiomer is active while the other one will do absolutely nothing, or will be less effective. In some cases, it is thus very important to be able to produce selectively one enantiomer and not the other one. Pharmaceutical indrustries invested such methods. If the reaction is not enantioselective, the productivity immediately drops by 50%. The optic activity of enantiomers also differs and is a good way to know which enantiomer have been produced.
Optic activity
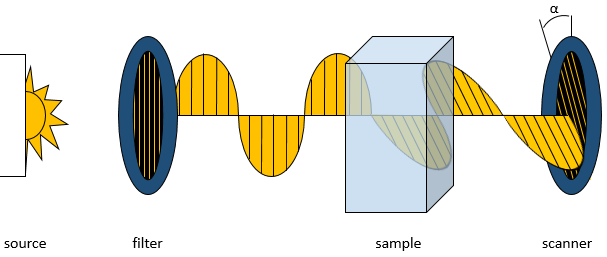
The optical activity of a compound is its influence on a plane polarised light beam. When the light, filtered only to oscillate in one plane, passes through a sample of an optically active compound, the beam is rotated by a given angle.
The angle of rotation depends on the molecules in the sample, of their concentrations and on the length of the sample cell. Each of these effects are linear and the modification of the angle of the light is given by the formula
The interest here is that enantiomers don’t have the same optical activity. The absolute modification of the angle is identical, but the direction in which the light is rotated is not. One enantiomer deviates the light towards the right and the other enantiomer deviates the light by the exact same angle but towards the left. The enantiomers are respectively defined as dextrogyre and levogyre and noted with a (+) or a (-).
The optical activity of an enantiomer is fixed for this molecule, at a given temperature t and for a light of given wavelength λ. Knowing its value, it is possible to determine the quantity of each enantiomer in a racemic mix/melange. A racemic melange is a solution containing the two enantiomers, not necessarily in the same quantity (nb: a more global definition of a racemic melange is the melange of several possible products of a single reaction). In such a melange, all the species will deviate the light with their normal effect.
If the two enantiomers are in equal quantities in the solution, the sample will be optically inactive, the effect on one enantiomer being counterbalanced by the effect of its specular image (i.e. the other enantiomer). If the quantities are not equal, the sample is optically active and the relative quantities of the enantiomers can be determined. The enantiomeric excess is the difference of proportion of the two enantiomers and is practically the proportion of the enantiomers that have an effect on the light. For example if the ratio between the enantiomers was 3:1 (75% of one (let’s say the dextrogyre) and 25% of the other enantiomer), the enantiomeric excess is 50%. Indeed, from the 75% of the dextrogyre enantiomer, the effect of 25% is counterbalanced by the levogyre enantiomer present in the solution. Only 50% of the (+) enantiomer deviates the light beam. If the pure enantiomer would have rotate the light plane by 26°, an entantiomeric excess of 50% rotates the light by 13° (50% of 26°).
Name of the enantiomers
We need a way to name differently the enantiomers. R or C will precede the name of the molecule and we will now see how to attribute which letter to which enantiomer. It is unfortunate but there is no clear correlation between the optic activity of an enantiomer and its structure. Another method had to be find.
The first step is to give a priority to each group attached to a stereocentre.
Priority is given in regard with the mass of the atom directly bound to the stereocentre. Let’s name these groups a,b,c and d by decreased priority (A priors to B, B priors to C, C priors to D). If two atoms have the same weight (two carbon are bound to the stereocentre for example), we look at the atoms they are wearing and, again, the priority goes to the carbon wearing the atom of larger atomic weight. If a methyl and an ethyl were bound to the stereocentre, the ethyl has the priority. Both groups are bound to the stereocentre by a carbon atom. We look now at the atoms on these carbons. The methyl has 3H and ethyl has 2H and one C. As C is heavier than H, the ethyl has the priority on the methyl.
Remember that it is the weight of the bound atom that matters, not the weight of the complete group. A –OH groups has indeed the priority on an ethyl group because O (atomic weight=16) is larger than C (12) even if the groups weight respectively 17 and 29 units of atomic mass. Note that we are talking about the mass of the elements. Isotopes can thus create stereocentres in molecules.
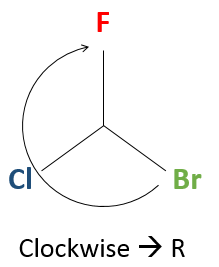
Next, we look at the molecule as if the group with the lowest priority (D) was behind the carbon. Generally the group with the smallest priority is a Hydrogen atom. This atom (or group) is not represented in the rest of the method.
So, looking at the stereocentre this way, we only see 3 liaisons connecting the stereocentre with the three groups of highest priority (A, B, C).
Now, we want to determine in which sense to rotate to go from the highest priority (A) to the lowest (C), passing by B. It may be helpful to place A in top of the representation.
If we must go clockwise, it is the enantiomer R. If it is counterclockwise, we are in presence of the enantiomer S.
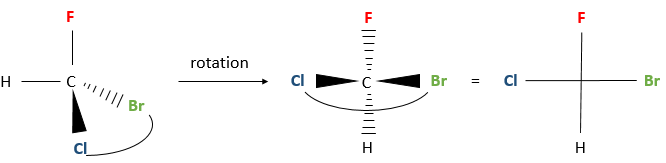
A second method exist, giving the same results, using the Fisher projection.
Instead of placing a group behind the stereocentre, we put two groups horizontally and two group vertically, still by a rotation of the stereocentre. The horizontally oriented groups are pointing toward the reader. The other two groups that are on the vertical axis are pointing opposite to the reader.
The rotation of the stereocentre is done by “grabbing” a pair or substituents and placing them in front of the molecule. Be careful to place the horizontal groups in the direction of the reader and the vertical ones in the opposite direction. Otherwise, a R enantiomer becomes S and vice versa.
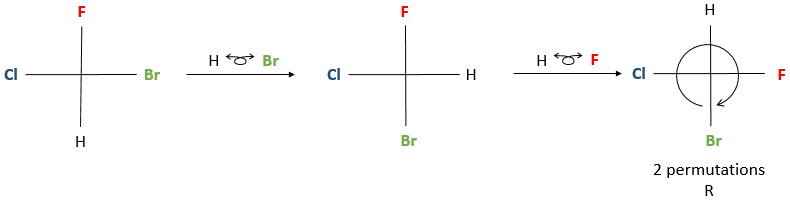
Once the rotation is done, to determine the correct configuration, the group of lowest priority has to be placed on the 12 o’clock position of the Fisher representation. Then, the configuration is determined the same way as for the Newman projection. If the lowest priority group was not in the top position after the rotation, don’t worry, we can perform permutations between near substituents. Performing one permutation changes the conformation of the enantiomer from R to S and vice versa. Performing two let the enantiomer identical.
If an even number of permutations (including zero) were done to put the lowest priority group in the 12 o’clock position, we can determine directly the configuration of the enantiomer. If an odd number of permutation was done, then you have two choices: either you determine the present configuration, knowing that the correct configuration is the other one, or you do one additional permutation and then determine the configuration.
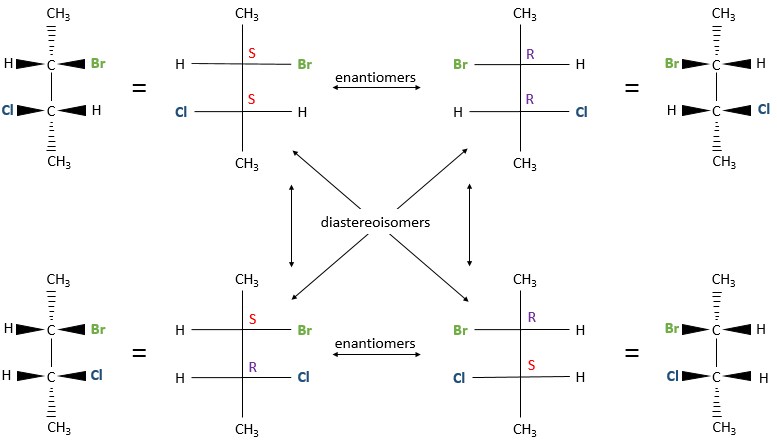
Diastereoisomers
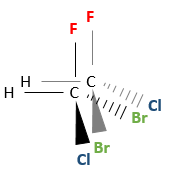
Several stereocentres may be present on a single molecule. The configuration of each stereocentre (R or S) is determined independently. If two stereocentres are on one molecule, several configurations are possible: RR, SS, RS and SR.
For example, 2-Bromo-3-chlorobutane has 4 stereoisomers.
All those conformations are not mirror image of each other. Some stereoisomers are enantiomers but some are not. Stereoisomers that are not the mirror image of each other are called diastereoisomer.
Exercises:
- Draw the skeleton structures of the isomers for C8H18. How many isomers did you find?
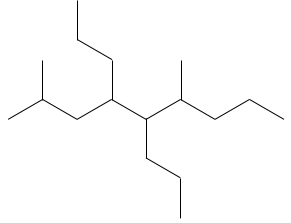
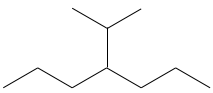
- Name the following molecules:
3. Draw the following molecule:
- 3,6,10-trimethyldodecane
- 5-ethyl-2-methyloctane
- 2,6-dimethyl-4,5-dipropylnonane
- 4-(1-methylethyl)heptane
- Is this name correct? If not, correct it.
- 2,5-dimethyl-4,6-dipropylnonane
- 3-ethyl-7-methyloctane
- 2,5-dimethyl-4-ethyldecane
- 4-(1-methylethyl)-5-propyldecane
- Draw the Newman projections of the C-C liaisons of this molecule and write if they are eclipsed or staggered
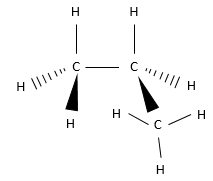
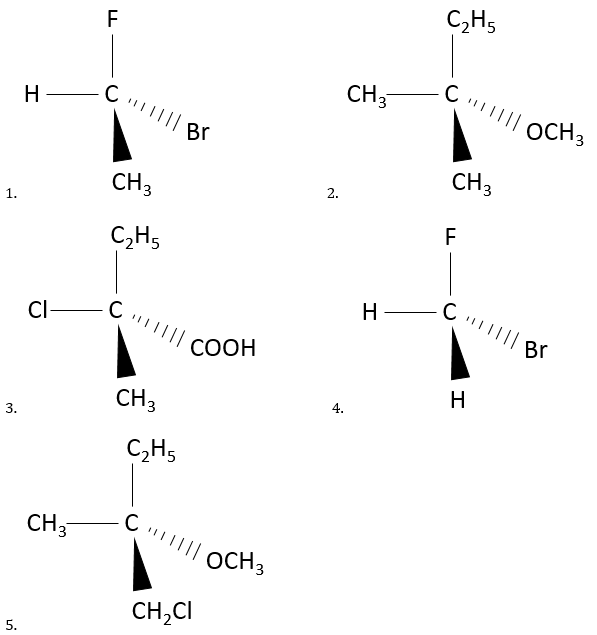
6. Chiral or achiral? If chiral, indicate if they are R or S.
Answers
1. There are 23 isomers of constitution for C8H18. A good way to find them all is to start from the longest main chain and decrease its length step by step.
2. Names:
2.1 n-hexane
2.2 Neopentane or 2,2-dimethylpropane
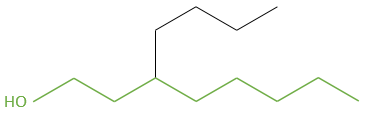
2.3 4-ethyl-7-methyldecane. It is not 4-methyl-7-ethyldecane because substituents are placed in alphabetical order if at the same distance from one extremity of the chain
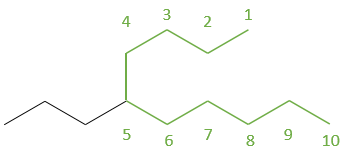
2.4 6-propyl-3-methyldecane (main chain from top to bottom right)
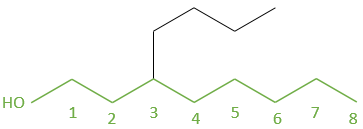
2.5 5-(1-methylpropyl)decane
2.6 4-ethyl-4-methyloctane
3. Names and sketch:
3.1. 3,6,10-trimethyldodecane
3.2. 5-ethyl-2-methyloctane
3.3. 2,6-dimethyl-4,5-dipropylnonane
3.4. 4-(1-methylethyl)heptane
4. Correct or not?
- 2,5-dimethyl-4,6-dipropylnonane: correct
- 3-ethyl-7-methyloctane: incorrect: the methyl substituent is closer of an extremity than the ethyl. The correct name is 6-ethyl-2-methyloctane
- 2,5-dimethyl-4-ethyldecane: incorrect: substituents have to be named in the alphabetical order. The correct name is 4-ethyl-2,5-dimethyldecane
- 4-(1-methylethyl)-5-propyldecane: correct
5. Newman projections going from left to right
6. Chirality:
6.1 Chiral: R
6.2 Achiral
6.3 Chiral: S
6.4 Achiral
6.5 Chiral: S