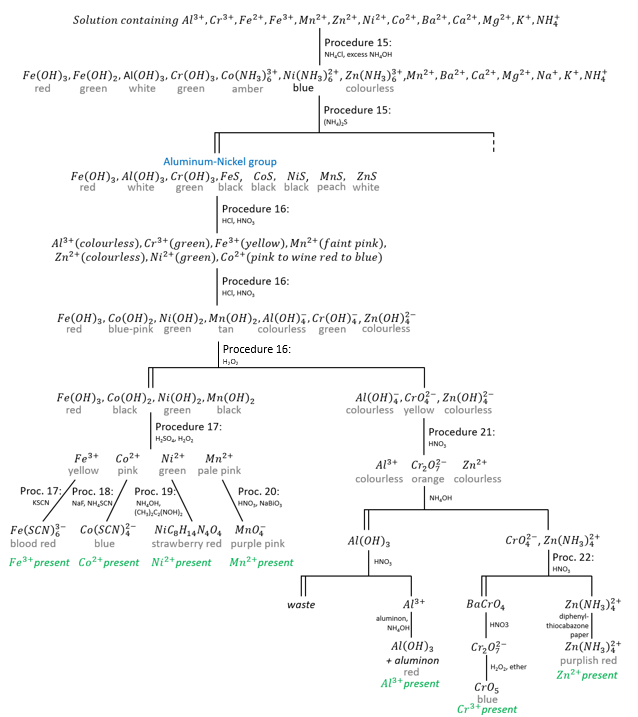
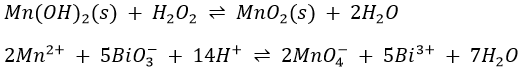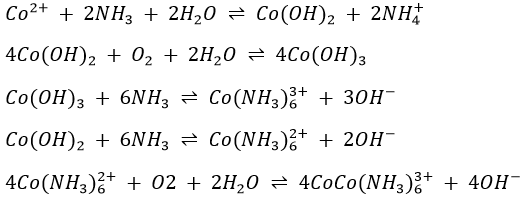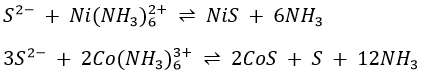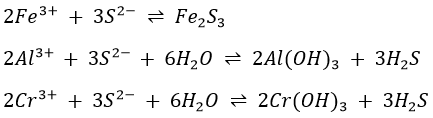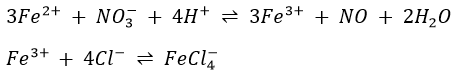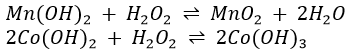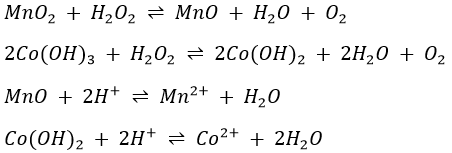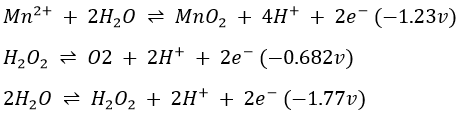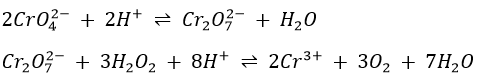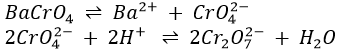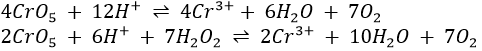Addition of NH4Cl, NH4OH and (NH4)2S to a solution containing all the cations not precipitated in the preceding groups results in the precipitation of aluminium, chromium and iron(II) as hydroxides, and manganese, nickel, cobalt, iron(II) and zinc as sulphides. Note that we used NH4OH and (NH4)2S in several tests of the preceding groups to separate ions or to confirm their presence.
The hydroxides and sulphides of calcium, barium, magnesium, potassium and sodium are soluble and belong to the barium-magnesium group.
Description of the elements of the silver group
Iron (Fe3+,Fe2+)
Iron in its compounds is ordinarily found in the 2+ (ferrous) or the 3+ (ferric) states. The latter is more common, since most ferrous compounds oxidize in the air, particularly if water is present. Iron(II) compounds are usually found as hydrates are light green. Iron(III) salts are also ordinarily obtained as hydrates are often yellow or orange. Both states form many complexes as Fe(CN)64- or Fe(CN)63-. Cyanide complexes are perhaps the most stable iron complexes and have characteristic deep red colours.
Metallic iron is a good reducing agent, dissolving readily in 6M HCl with evolution of hydrogen. Conversion of Fe(II) to Fe(II) or the reverse is easily accomplished by common oxidizing agents (air of H2O2 in acid) or by reducing agents such as H2S, Sn2+ or I–.
Cobalt, Co2+
Like iron, cobalt can exist in two common oxidation states in aqueous solutions, Co2+ and Co3+. The 3+ state is stable only in presence of strong coordinating liquids such as NH3; the general chemistry of cobalt schemes of qualitative analysis is usually that of Co2+.
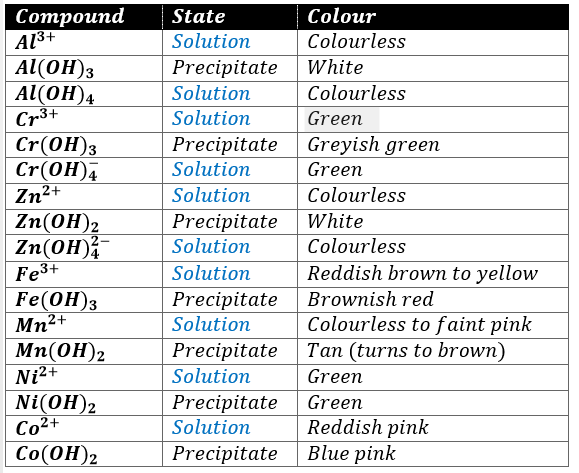
Cobalt(II) salts in water solution are characteristically pink, the colour of the hydrated cobalt ion Co(H2O)62+, but the colour is too delicate to be used to characterise the ion. Cobalt(II) forms several complex ions. Cobalt chloride solution turns from pink to blue when heated to boiling because of a change in coordination number: the pink form arises from octahedrally coordinate Co(II), whereas the blue form is tetrahedrally coordinated Co(II). Cobalt sulphide does not dissolve readily in 6M HCl even when heated. Cobalt sulphide does not dissolve readily in 6M HCl even when heated. Co(II) reacts with thiocyanate solutions to form a blue complex, Co(SCN)42-, which is much more stable in ethanol than in water. Addition of KNO2 to solutions of Co2+ produces a characteristic yellow precipitate of K3Co(NO2)6 in which the metal has been oxidized:
Under strongly oxidizing conditions, Co(II) can be converted to Co(III), which has a stability that is enhanced in a complex species like Co(NH3)63+ or an insoluble substance such as the yellow cobalt nitrite produced in the reaction above.
Nickel, Ni2+
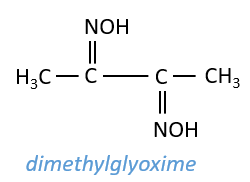
Nickel salts, like those of most of the other members of group III, are typically coloured: hydrates are green, Ni(NH3)62+ is blue and many other nickel complexes have characteristic colours, and nickel forms a very characteristic rose red precipitate with dimethylglyoxime (N2C4H8O2, structure shown below), an organic chelating agent.
Chromium, Cr3+
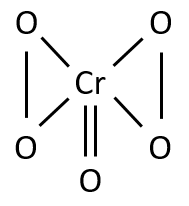
The common oxidation states of chromium are 3+ and 6+. Cr3+ forms several complexes, all of which are coloured. Cr(H2O)63+ is reddish violet in solution. The chromium-containing precipitate obtained in the group III precipitation scheme is the hydroxide Cr(OH)3 rather than the sulphide. Chromium(III) hydroxide is amphoteric: it dissolves in excess base to form the green chromite Cr(OH)4– and in acid to form the hydrate chromium(III) ion. Chromium(III) can be oxidized to Cr(VI) with several oxidizing agents, such as ClO3– in 16M HNO3, H2O2 in 6M NaOH, and ClO– in 6M NaOH. Chromium is usually identified in the form of a Cr(VI) species in neutral or basic solutions. CrO42- is bright yellow and if acid is added, the orange Cr2O72- ion is formed.
If H2O2 is added to a solution containing the dichromate ion, a transitory blue colour due to CrO5 is observed. It is the basis for the confirmatory test for chromium. The following structure has been proposed for CrO5.
The oxidation number in CrO5 can be calculated to be 10. However, it does not mean that 10 electrons are involved in the bonding.
Aluminium, Al3+
In aqueous chemistry, the only important oxidation state of aluminium is the 3+ state. All its simple salts are colourless (Al is not a member of the transition series of elements). Most aluminium compounds are soluble in water (exceptions: its oxide and hydroxide). The hydrated ion Al(H2O)63+ is colourless too. Aqueous solutions of aluminium salts are extensively hydrolysed and are acidic.
In extreme cases, hydrolysis can be sufficiently extensive to yield cloudy solutions containing hydrated hydroxides. The suspension can be reversed by making the solution acidic, reversing the chemical equation.
Manganese, Mn2+
The aqueous chemistry of manganese incorporates several common oxidation states: Mn(II), Mn(IV) and Mn(VII). Aqueous solutions of Mn2+ are coloured pink. MnO2 is insoluble in water and KMnO4 is deeply purple. Mn(II) can be oxidized to Mn(IV) in basic solution but with stronger oxidizing agents in acid solution, oxidation to Mn(VII) can occur.
We don’t make confirmatory tests based on the pink colour of Mn(II) but well on the characteristic colour of the permanganate ion.
Zinc, Zn2+
Compounds containing the Zn2+ ion are usually colourless and many are soluble in water. The ion complexes to for the colourless Zn(H2O)42+ which can be hydrolysed following the equation:
Other complexes can be formed with NH3 or OH–. Zinc hydroxide is amphoteric, dissolving in both acid and base.
Precipitation and analysis of the aluminium-nickel group
The conditions for the precipitation of the aluminium-nickel group of ions must be carefully maintained to keep ions from the following groups from precipitating prematurely. The most problematic ion is Mg2+ as Mg(OH)2. We avoid its precipitation by manipulating the equilibrium associated with NH4Cl. NH3 is a weak base that ionizes to give enough OH– to form a precipitate with Mg2+ if it is present in a solution. Ammonium chloride is a salt and hence a strong electrolyte. Consequently, NH4Cl provides NH4+ ions to keep the equilibrium
to the left. There won’t be enough OH– ions to form a precipitate of Mg(OH)2. Ammonium chloride also prevents the precipitation of Mn(OH)2 in exactly the same way, but MnS is quite insoluble so that manganese precipitates as MnS when (NH4)2S is added, while MgS is soluble and does not precipitate.
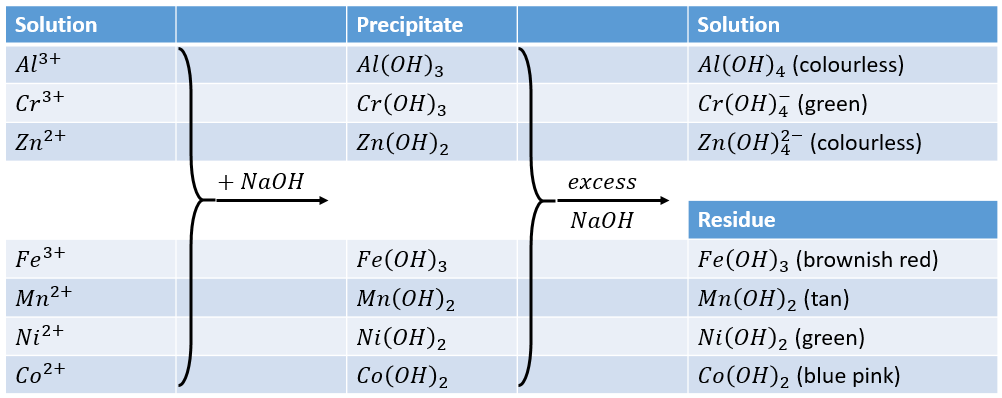
The hydroxides of aluminium, chromium and iron(III) are so insoluble that they precipitate even in the presence of the low concentration of OH– even in presence of NH4Cl. The hydroxides of cobalt, nickel and zinc are likewise so insoluble that they also will precipitate but they redissolve in excess NH4OH. The behaviour of iron(II) is complicated in aqueous solution because it is readily oxidized by air to Fe(III).
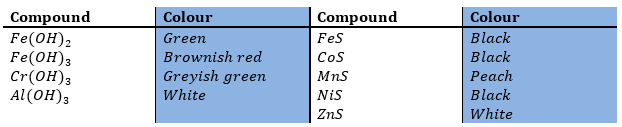
A solution containing Fe(OH)2 will initially show a green precipitate that gradually darkens as more Fe(OH)2 is oxidized to Fe(OH)3, brownish red.
The colour of the precipitate can thus give precious information about the presence or absence of certain ions.
If NH4Cl and aqueous NH3 give no precipitate, aluminium, chromium and iron(II and III) are definitely absent. In interpreting colours, we must recognize that a dark colour will obscure or cover up a lighter colour. Fe(OH)3 can thus cover up Cr(OH)3 or Al(OH3).
If the addition of (NH4)2S gives no further precipitation, cobalt, nickel, manganese and zinc ions are absent. A black precipitate does not give a good clue on which ions are present but a peach or white precipitate indicate respectively the presence of manganese and of zinc.
The hydroxide of iron is converted to the black sulphide.
The interest of the separate additions of NH4Cl and (NH4)2S is the observation of the colours of the precipitates.
The behaviours of zinc, nickel and cobalt ions when treated with aqueous NH3 is the same as that already noted for copper and cadmium ions. The hydroxides of the three metals are first formed. They are dissolved by excess NH4OH to form Zn(NH3)42+, Ni(NH3)62+ and Co(NH3)63+, respectively colourless, pale violet blue and deep amber. Note that cobalt(II) has been oxidized to cobalt(III) in the process: in alkaline solutions, cobalt(II) is a fairly strong reducing agent and is slowly oxidized to cobalt(III) by atmospheric oxygen.
Ammonium sulphide reacts with the Zn(NH3)62+, Ni(NH3)62+ and Co(NH3)63+ complexes to form the corresponding sulphide ZnS, NiS and CoS.
Also, MnS and FeS are formed by direct combination of the two ions.
Ferrous sulphide may also be formed by reduction of Fe(III) as described by (*).
If (NH4)2S is added to a cold neutral solution containing aluminium, chromium and iron(III) ions, Al(OH)3, Cr(OH)3 and Fe2S3 will be precipitated.
At higher temperatures, Fe2S3 is hydrolysed, giving a brownish red precipitate.
and FeS is formed (reaction (*)).
Procedure 15
The decantate from Procedure 5 is placed in a casserole and is evaporated down to a volume of 8 to 10 drops. Transfer to a test tube, centrifuge and decant into another test tube, discarding the precipitate. To the decantate, add 4 drops of 2M NH4Cl, mix thoroughly, and then add 15M aqueous NH3 drop by drop and with constant stirring, until the solution is just alkaline. Then add one more drop and 20 drops of hot water. Mix thoroughly, centrifuge but do not decant. Note the colour of the precipitate and of the supernatant liquid. Add 8 or 9 drops of ammonium sulphide and mix again.
Heat the tube carefully for one or two minutes in the boiling water bath, taking care to avoid any overflow. Centrifuge and test for complete precipitation with a drop of ammonium sulphide. When we are sure that the precipitation is complete, wash down the sides of the tube with a few drops of hot water, centrifuge and note the colours of the precipitate and of the supernatant liquid, then decant, saving the decantate for Procedure 2’. Wash the precipitate 3 times with 20-drops portions of a hot solution prepared by mixing equal volumes of water and 1M NH4C2H3O2 and analyse according to Procedure 16.
Notes
1. The decantate from the copper-arsenic group is boiled down before the aluminium-nickel group is precipitated in order to drive off all H2S and to precipitate any sulphide of the copper-arsenic group that may have gone in the decantate. If the H2S were not removed, the sulphides and hydroxides of the aluminium-nickel group would all precipitate together when aqueous NH3 was added. As a result, the desirable valuable observation of colours of precipitates to which we referred earlier could not be made.
2. If phosphate, borates, fluorides, oxalates, silicates or tartrates are present in the solution and that the solution also contains calcium, barium, or magnesium ions as well as cations of the aluminium-nickel group, a special series of procedures must be used instead of the ones presented in this section. However, the combination of ions just mentioned is rare.
3. NH4Cl is a strong electrolyte that helps coagulate any hydroxides or sulphides, thereby preventing them from becoming colloidal. Similarly, the addition of NH4C2H3O2 to the washing solution prevents peptization of the precipitate being washed.
4. NH3 is added drop by drop and the pH is verified because a large excess of NH4OH tends to cause dispersion of the precipitated sulphides and hydroxides. If it is difficult for the precipitates to settle down, the mixture should be boiled for one minute before centrifuging.
Separation of the aluminium subgroup from the nickel group
The hydroxides of aluminium, chromium and zinc are amphoteric and are, therefore soluble in NaOH. In contrast, the hydroxides of iron, manganese, cobalt, and nickel are not amphoteric and therefore not soluble in NaOH.
Of the species precipitated as the aluminium-nickel group of ions, Al(OH)3, Fe(OH)3, Cr(OH)3, MnS, FeS and ZnS are readily soluble in HCl while NiS and CoS are not. However they are soluble in aqua regia (HCl+HNO3).
Aqua regia oxidises Fe(II) to Fe(III).
When NaOH is added to a solution containing the members of the aluminium-nickel group, the hydroxides of all seven metals are first precipitated. The hydroxides of aluminium, chromium, and zinc are amphoteric and dissolve in an excess of NaOH to form the complex ions Al(OH)4–, Cr(OH)4–, and Zn(OH)42-, respectively. The hydroxides of iron, manganese, cobalt, and nickel are not amphoteric and do not dissolve in excess NaOH.
A careful observation of colours of solutions and precipitates formed in Procedure 16 may give information regarding the cations present.
The precipitate formed when NaOH is added to Co2+ may vary in colour from blue to pink to tan to light brown. Co(OH)2 exists in two forms, one blue (believed to be more dispersed) and one pink. It changes to the coarser pink form on standing and heat speeds up the process. Air oxidizes Co(OH)2 to black Co(OH)3. A pink solution of CoCl2 turns blue when heated but regains its pink colour when cooled. It also turns blue when treated with concentrated HCl and the pink colour is restored by dilution with water. These colour changes are due to changes in the composition and structure of the complex ions present in the solution. Pink is due to Co(H2O)62+ and blue to Co(H2O)Cl3– and CoCl42- ions. The colours of all the solution given above are probably due to similar complex ions.Pure, freshly precipitated Mn(OH)2 is tan but it turns brown in contact with air when MnO2 oxidises into Mn2O3.
The fact that nickel and cobalt do not precipitate in the copper-arsenic group indicates that NiS and CoS are soluble in dilute HCl. In the analysis of the aluminium-nickel group, however, CoS and NiS do not dissolve in concentrated HCl. This contradictory behaviour is explained as follows: freshly precipitated NiS and CoS exist in a form readily dissolved by dilute HCl. On standing, this soluble modification changes into a second form that is insoluble in both dilute and concentrated HCl.
Separation of the aluminium subgroup from the nickel subgroup
Procedure 16
Treat the precipitate from Procedure 15 with 10 drops of 12M HCl, mix thoroughly, transfer to a casserole, and boil gently for 30s. If the precipitate is not completely dissolved, add 3 drops of 16M HNO3, mix thoroughly, and boil until a clear solution is obtained. Add 10 drops of cold water, transfer to a test tube, centrifuge to remove any precipitate of sulphur, and decant into a casserole. Note the colour of the solution. Make the solution strongly alkaline with 8M NaOH and mix thoroughly. If the quantity of precipitate is so large that the product is mushy or non-fluid, add 10 to 20 drops of water. Note the colour of the solution and of the precipitate. Then add 2 drops of 3% H2O2, stir for one minute and then boil for two minutes, replenishing the water lost. Transfer to a test tube before the precipitate has had a chance to settle and centrifuge. Decant, saving the decantate for Procedure 21. Note the colour of the precipitate and of the decantate. Wash the precipitate three times with hot water and analyse according to Procedure 17.
Notes
1. Hydrogen peroxide is added to oxidize chromate(III) (Cr(OH)4–, green) to chromate(VI) (CrO42-, yellow).
If chromium is present, the colour of the solution will thus change. A blackening of the precipitate may indicate the formation of MnO2 or Co(OH)3.
2. Any excess of H2O2 present in the solution must be decomposed by boiling; otherwise it will interfere with Procedure 21.
Detection of iron, cobalt, nickel and manganese
Up to this point in the analysis, the general practice has been to separate a cation from all other cations before identifying it. The exceptions were Cu2+ and Cd2+, which were identified in each other’s presence, and Sb3+, which was identified in the presence of Sn4+.
In the identification of Fe3+, Co2+, Ni2+ and Mn2+, separate samples of the same solution will be used in making each confirmatory test; the four ions will not be separated from each other prior to the test. This can be done because in this particular quartet, none of any three interferes seriously with the confirmatory test for the fourth.
Fe(OH)3 and Ni(OH)2 dissolve readily in 2M H2SO4 to give Fe3+ and Ni2+, but MnO2 and Co(OH)3 are very slowly dissolved by 2M H2SO4. Hydrogen peroxide speeds up the solution process by reducing MnO2 and Co(OH)3 to MnO and Co(OH)2. These latter compounds, in which Mn and Co are in their lower oxidation states; are more basic in character and as a result are readily dissolved by H2SO4. The net equations for the reduction by H2O2 and the subsequent solution by H2SO4 are
Note that in basic solutions, H2O2 oxidizes Mn(OH)2 and Co(OH)2 to MnO2 and Co(OH)3 (Procedure 16), what is the opposite that we seek here. In Procedure 17, H2O2 functions as a reducing agent and reduces MnO2 and Co(OH)3 to Mn2+ and Co2+, respectively. The role H2O2 takes (reducing or oxidizing agent) depends on the relative reducing and oxidizing power of the substance with which it reacts and the relative rates of the reactions involved. In basic solutions, the potential of the half reaction
is only slightly lower than that of the half reaction
but very much higher than that of the half-reaction
Accordingly, the tendency for H2O2 to oxidize Mn(OH)2 to MnO2 will be very much greater than its tendency to reduce MnO2 to Mn(OH)2. This is equivalent to saying that, in basic solution, the reducing power of Mn(II) toward H2O2 is much greater than the oxidizing power of Mn(IV) toward H2O2. In acid solutions, the situation is essentially reversed.
Although the potential of the first half reaction differs from that of the second by about the same amount it does from that of the third, the rate of the reaction of H2O2 involving the former is considerably greater than that of the latter. As a result, the tendency for H2O2 to reduce MnO2 to Mn2+ is greater than its tendency to oxidize Mn2+ to MnO2. The same rationale applies to the behaviour of Co(II) and Co(III).
In an acid solution, H2O2 will thus not oxidize Co2+ to Co3+, but the tendency for Co3+ to be reduced to Co2+ will be great. In basic solution, the potential of the half reaction
is -0.17v. Accordingly, the tendency for H2O2 to oxidize Co(II) to Co(III) will be very much greater than its tendency to reduce Co(III) to Co(II). During the reactions, molecules of O2 gas are formed.
Procedure 17
To the precipitate from Procedure 16, add 20 drops of 2M H2SO4, mix thoroughly, and transfer to a casserole. Boil gently for one minute, add a drop of 3% H2O2, and continue boiling for one minute after the precipitate is completely dissolved. Add 10 drops of water, allow to cool, note the colour of the solution, and divide into four approximately equal portions to use to test for iron, cobalt, nickel and manganese.
Test for iron: to one portion, add one or two drops of 0.2M KSCN. A blood red solution, due to Fe(SCN)63+ proves the presence of iron.
Notes
1. To test for iron in a solid, proceed as follows: dissolve a small sample of the solid in 10-15 drops of dilute HCl. Divide the solution into three parts. Test one part for iron(III) ions according to Procedure 17. To the second part, add a few drops of potassium hexacyanoferrate(II), K4Fe(CN)6. A dark blue precipitate (Prussian blue) proves the presence of Fe3+ ions.
To the third part, add a few drops of potassium hexacyanoferrate(III), K3Fe(CN)6. A dark blue precipitate (Turnbull’s blue) proves the presence of Fe2+ ions.
Note that the oxidation states are different.
Although the Fe(SCN)63- ion is widely accepted as the predominant species responsible for the red colour in the test for Fe3+, there is little doubt that complexes with a smaller number of SCN–, such as FeSCN2+, Fe(SCN)2+, …, are present too. When the concentration of SCN– is high, as in the case when 0.2M KSCN is added in making the test, the predominant species is likely to be Fe(SCN)63-.
Detection of cobalt
Procedure 18
To the second portion of the solution prepared in Procedure 17, add enough solid NaF to form a saturated solution; mix well by stirring. Then add 10-20 drops of a saturated solution of NH4SCN in ethyl alcohol. The formation of a blue solution due to Co(SCN)42- proves the presence of Co2+.
Notes
1. The identification of Fe3+ and Co2+ in Procedures 17 and 18 is unique in that each is identified by the use of the same reagent and each forms the same type of complex ion, Fe(SCN)63- and Co(SCN)42-. The test for iron is carried out in dilute aqueous solution, in which the red complex ion Fe(SCN)63- is very stable; the blue complex ion Co(SCN)42- is unstable in aqueous solution. As a result, cobalt ions do not interfere with the test for iron. The test for cobalt is carried out in alcoholic solution and in the presence of fluoride ions. The blue complex ion Co(SCN)42- is stable in alcoholic solution; the Fe3+ ions are tied up in the form of the colourless stable ion FeF63- (or, possibly, FeF2+, FeF2+, FeF4–, or FeF32-) and cannot, therefore, interfere with the test for cobalt.
2. The reaction of Co2+ with SCN– is incomplete, due to the instability of the complex ion.
An excess of SCN– is necessary to give a good test.
Detection of Nickel
Procedure 19
Make the third portion of the solution prepared in Procedure 17 basic with 5M aqueous NH3. If a precipitate of Fe(OH)3 or Mn(OH)2 forms, centrifuge and decant; to the clear decantate, add 2-4 drops of dimethyl-glyoxime, mix thoroughly, and allow to stand for one minute. A strawberry red precipitate (NiC8H14N4O4) proves the presence of nickel.
Notes
1. The following reaction takes place in the confirmatory test for nickel:
Since the red compound is readily soluble in acids, the reaction must be carried out in alkaline solution. Iron(II) ions react with (CH3)2C2(NOH)2 to form a soluble red compound; however, no precipitate is formed.
Detection of Manganese
Procedure 20
Dilute the fourth portion with an equal volume of water, add 2 drops of 3M HNO3, mix thoroughly, then add a few grains of solid sodium bismuthate, mix thoroughly, and allow to stand for 1min. Centrifuge. A pink to reddish purple solution, due to the permanganate ion (MnO4–) proves the presence of manganese.
Notes
1. Bismuth is predominantly metallic in character; its common oxidation number is 3 and its compounds ionize to give Bi3+ In the compound NaBiO3, bismuth has an oxidation number of 5 and has the property of a non-metal in that it is present in the negative ion BiO3–. This behaviour of bismuth illustrates the rule that as the oxidation number of an element increases, it becomes more non-metallic in character.
The behaviour of NaBiO3 illustrates the rule that those compounds of polyvalent elements in which the element exists in one of its higher oxidation states generally can act as oxidizing agents. Other compounds (oxidizing agents) that illustrate this rule are KMNO4 and K2Cr2O7.
2. The oxidation of Mn2+ to MnO4– by sodium bismuthate (NaBiO3) takes place as follows:
Reducing agents of any kind, such as chlorides and sulphides, will interfere with the confirmatory test for manganese because they reduce the violet MnO4– to the practically colourless Mn2+.
Separation and detection of aluminium
Procedure 21
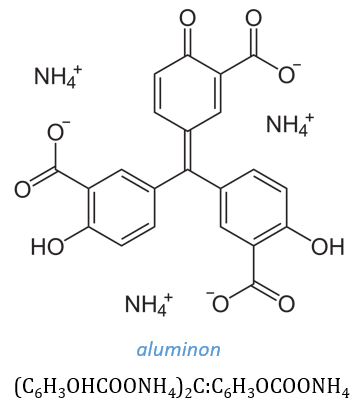
Treat the decantate from Procedure 16 with 16M HNO3 until slightly acid; then add 15M aqueous NH3 until distinctly alkaline. Continue stirring the ammoniac solution for 1 min. Centrifuge and decant, saving the decantate for Procedure 22. Because the Al(OH)3 that precipitates is gelatinous, highly translucent, very finely divided, and the colour of opaque, bluish white glass, its presence, suspended in the solution, is not easy to detect. On centrifuging, however, it will appear in the bottom of the test tube as a whitish, jellylike, opaque precipitate. Wash the precipitate three times with hot water, then dissolve, remove it by centrifuging and decantation. Add 2 drops of aluminon solution, mix thoroughly, make just barely alkaline with 5M aqueous NH3, again mix thoroughly, and then centrifuge. A cherry red precipitate proves the presence of aluminium.
Notes
1. It is imperative that all chromate(III) ions (Cr(OH)4–) be completely oxidized to chromate(VI) ions (CrO42-) in Procedure 16. Any unoxidized Cr(OH)4– will react as follows:
This reaction of chromate(III) ions is the same as that which the aluminate ion (Al(OH)4–) will undergo in the course of the separation.
It is thus evident that if Cr(OH)4– is not oxidized to CrO42-, Cr(OH)3 will precipitate when the test for aluminium is made. It is obvious, therefore, that oxidation of Cr(OH)4– to CrO42- by H2O2 is necessary if a separation of chromium from aluminium is to be accomplished. Although Cr(OH)3 is green, in small amounts the colour is not marked, and the Cr(OH)3 precipitate may be mistaken for Al(OH)3.
Cr(OH)3 does not form a red lake with aluminon. Excessive amounts of Cr(OH)3 (green) will, however, mask the red aluminon. Therefore, if much Ce(OH)3 precipitates with the Al(OH)3, it should be reoxidized to CrO42- with H2O2.
2. When HNO3 is added in the first step in Procedure 21, a precipitate sometimes forms when the solution is just about neutral and then redissolves when more HNO3 is added. This precipitate may be either Al(OH)3 or Zn(OH)2, both of which are white; in case chromium is not completely oxidized to CrO42-, Cr(OH)3 (green) mau precipitate. Two reactions account for the formation and disappearance of this precipitate.
a) When HNO3 is added to neutrality,
b) When more HNO3 is added,
Excess H2O2, if present in the decantate from Procedure 16, will interfere with the separation and identification of aluminium and chromium. When HNO3 is added in the first step in Procedure 21, the following reactions will take place.
The chromate is thus reduced to Cr3+, which will then interfere with the test for aluminium is already discussed in Note 1.
3. When NH3 + 3H2O is added in Procedure 21, a white precipitate sometimes forms; it redissolves when more aqueous NH3 is added. This precipitate is probably Zn(OH)2. The reactions that account for its formation and redissolution are
4. Since HCl may reduce chromate to Cr3+, the solution is acidified with HNO3 rather than with HCl.
5. If lead, tin, and antimony are not completely precipitated in the copper-arsenic group, they appear as insoluble hydroxides or basic salts in the final test for aluminium. The hydroxides, like Al(OH)3, do not dissolve in excess NH4 They do not, however form the characteristic red lake with aluminon.
6. Aluminon is an organic dye. It is the ammonium salt of aurintricarboxylic acid and its chemical formula is
7. A lake is formed when a coloured compound (a dye) combines with or is adsorbed from a solution by an insoluble gelatinous precipitate. The dye aluminon is preferentially adsorbed by al(OH)3: this dye is not adsorbed by Cr(OH)3 or Zn(OH)2 or by the hydroxides of lead, tin, and antimony.
Separation and detection of chromium and zinc
Procedure 22
Follow (A) is the decantate from Procedure 21 is colourless, follow (B) if it is yellow.
(A) Decantate; therefore chromium is absent. Place a drop of the decantate on a piece of diphenylthiocarbazone paper and allow to stand for 1 min. A purplish red coloration proves the presence of zinc ions (in the absence of the Zn2+ ion, NH4OH will give a yellowish brown colour). A confirmatory test for Zn2+ is given in Note 5.
(B) Decantate is yellow; chromium is probably present. Add 6-7 drops of 0.2M BaCl2 to the yellow decantate, mix thoroughly, centrifuge until the supernatant liquid is clear, and decant, saving the decantate for (C). Wash the precipitate, BaCrO4 mixed with some BaSO4, twice with hot water, ad 3 drops of 3M HNO3, heat gently but do not boil vigorously, and stir for about 1 min. Add 10 drops of cold water, mix thoroughly, cool under the cold water tap, and then ad 10 drops of ether and 1 drop of 3% H2O2. Mix well by vigorous stirring and allow to settle. A blue coloration of the ether layer due to chromium peroxide (CrO5) proves the presence of chromium.
(C) Place a drop of the decantate from (B) on a piece of diphenylthiocarbazone paper and allow to stand for about 1 min. A purplish red coloration proves the presence of zinc ions (in absence of Zn2+ ions NH4OH will give a yellowish brown colour). A confirmatory test for Zn2+ is given in Note 5.
Notes
1. The final test for chromium depends on the fact that in dilute acid solution Cr2O72- interacts with H2O2 to form a deep indigo blue compound, chromium peroxide(CrO5). Yellow chromate is first changed to orange dichromate (Cr2O72-).
This change of chromate to dichromate, which always take place when the solution is acidified, has already been mentioned in Note 2, Procedure 21.
The dichromate is then converted to CrO5 by H2O2.
The compound CrO5 is unstable and decomposes on standing to form Cr3+. If the concentration of HNO3 is low, the CrO5 decomposes very slowly; if, however, the concentration of HNO3 is high, CrO5 decomposes so rapidly that the blue colour may not be noticed. Furthermore, CrO5 is very rapidly reduced to Cr3+ by excess H2O2. Hence high concentrations of HNO3 and excess H2O2 must be avoided.
CrO5 is very soluble in ether; HNO3 is not. Treating with ether partially separates the CrO5 from HNO3 and concentrates it in the ether layer. Since separation from HNO3 will increase the stability of CrO5, extraction with ether preserves as well as concentrates CrO5.
2. The precipitate obtained on addition of BaCl2 may contain varying amounts of BaSO4 because of oxidation of H2S and S to H2SO4 in earlier procedures. BaSO4 is white, whereas BaCrO4 is light yellow. Very finely divided BaCrO4 is such a pale yellow, however, that the precipitate may appear white even though it consists largely of BaCrO4. The confirmatory test for chromium should therefore be completed as directed, even if the precipitate appears white.
BaCrO4, being the salt of the relatively weak acid H2CrO4, is soluble in HNO3, whereas BaSO4, being the salt of a strong acid, is insoluble.
3. Diphenylthiocarbazone paper is also called dithizone paper.
4. The purplish red colour is believed to be due to the complex ion formed by interaction Zn2+ ions with diphenylthiocarbazone molecules, which have the following structure.
5. Make the decantate from (B) acid with 6M HCl. Then add 3-4 drops of 0.2M K4Fe(CN)6 and mix thoroughly. The resulting mixture should be acidic. A greyish white to bluish green precipitate, Zn3K2[Fe(CN)6]2, proves the presence of zinc.