Introduction to bioenergetics
Cellular respiration is composed of three topics:
- Redox reactions (oxidation-reduction)
- Electron transport chain
- Glycolysis
In fact living cells require energy from outside sources. Some animals obtain energy by eating plants and some others feed on other organisms that eat plants, and plants receive energy from sunlight. we can resume, the situation:
Energy flows into an ecosystem as sunlight and leaves as heat.
↓
Photosynthesis generates O2 and organic molecules which are used in cellular respiration.
↓
Cells use chemical energy stored in organic molecules to regenerate ATP, which powers work.
These Catabolic pathways resulting in production of ATP are exergonic (exergonic means energy is released during the process).
Cells do this by different ways.
- Fermentation is a partial degradation of sugars that occurs in the absence of O2 .
- Aerobic respiration is really cellular respiration: it consumes organic molecules and O2 and yields ATP.
- Anaerobic respiration is similar to aerobic respiration but consumes compounds other than O2 .
Redox reactions (oxidation and reduction) involves:
Transfer of electrons during chemical reactions and release energy stored in organic molecules.
The released energy is ultimately used to synthesize ATP.
The principle of redox
Chemical reactions that transfer electrons between reactants are called oxidation-reduction reactions, or redox reactions.
In oxidation, a substance loses electrons, or is oxidized.
In reduction, a substance gains electrons, or is reduced (the same amount of positive charge is reduced)
Example: NaCl → Cl- + Na+
Na= oxidized
Cl = reduced
We can wright it in a general way:
reducing agent + oxidizing agent → reduced agent + oxidized agent
The electron donor is called the reducing agent and the electron receptor is called the oxidizing agent.
Some redox reactions do not transfer electrons but change the electron sharing in covalent bonds.
An example is the reaction between methane and O2
Energy
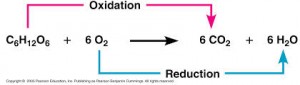
During cellular respiration, the fuel (such as glucose) is oxidized, and O2 is reduced:
Oxygen is a very electro negative agent.
The glucose is the fuel of the organism but the reaction does not happen in one step as shown here and it happens during several steps, this is a very exergonic reaction and the ΔG =-686 Kcal/mol.
The glucose is around and O2 also, why there is no spontaneous combustion? Because the activation energy is high.
stepwise energy harvest via NAD+ and the electron transport chain.
-In cellular respiration, glucose and other organic molecules are broken down in a series of steps.
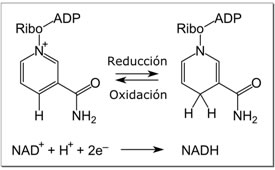
-Electrons from organic compounds are usually first transferred to NAD+ a coenzyme.
-As an electron acceptor,NAD+ functions as an oxidizing agent during cellular respiration.
-Each NADH (the reduced form of NAD+ ) represents stored energy that can be tapped to synthesize ATP.
CH3OH + NAD+ → NADH + CH2O + H+
-NADH passes the electrons to the electron transport chain.
-Unlike an uncontrolled reaction, the electron transport chain passes electrons in a series of steps instead of one explosive reaction.
-O2 pulls electrons down the chain in an energy-Yielding Tumble
-The energy yielded is used to regenerate ATP.
The stages of cellular respiration: A preview stages
Cellular respiration has three stages:
- Glycolysis (breaks down glucose into two molecules of pyruvate)
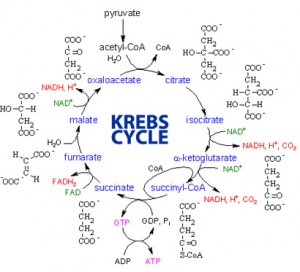
- The citric acid cycle (completes the breakdown of glucose)
- Oxidative phosphorylation (accounts for most of the ATP synthesis)
Glucose→NADH→electron transport chain→H2O + CO2
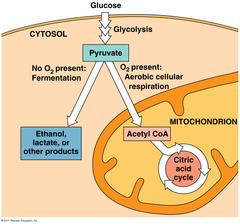
Glycolysis is occurring in the cytosol.
And when pyruvate is produced, it enters the citric acid cycle which is going on inside of the mitochondria.
The process that generates most of the ATP is called oxidative phosphorylation because it is powered by redox reactions.
Oxidative phosphorylation, accounts for almost 90% of the ATP generated by cellular respiration.
A smaller amount of ATP is formed in glycolysis and the citric acid cycle by substrate-level phosphorylation.
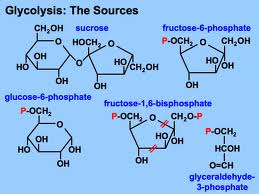
Glycolysis harvests chemical energy, by oxidizing glucose to pyruvate.
Glycolysis (“splitting of sugar”) breaks down glucose, into two molecules of pyruvate.
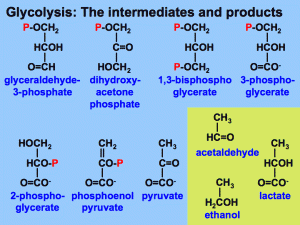
Glycolysis occurs in the cytoplasm and has two major phases:
- Energy investment phase
- Energy payoff phase