AEROBIC WAY
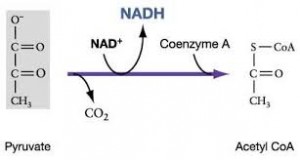
The citric acid cycle completes the energy yielding oxidation of organic molecules. In the presence of O2, pyruvate enters the mitochondrion. Before the citric acid cycle can begin, pyruvate must be converted to acetyl CoA which links the cycle to glycolysis
The first step in this process, is that before the citric acid cycle can actually going, pyruvate has to go across the mitochondrial membrane and just after entering it reacts, with a large enzyme complex, called Pyruvate dehydrogenase (PDH) and that enzyme complex catalysis three steps:
- 1st step: carbon dioxide (CO2) is released
- 2nd step: we have the reduction of NAD+ molecule to NADH
- 3rd step: this reaction is with coenzyme A
Coenzyme A has 3 SH and when it reacts with acetate (pyruvate -Co2),it gives acetyl CoA and it is a high energy bound
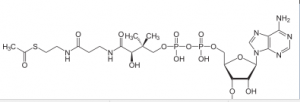
This is a critical step because it allows this molecule to be at the top of an energy hill and goes downhill in the subsequent reaction. pyruvate is a charged molecule so it cannot freely cross the mitochondrial membrane and in fact it must be transported across the membrane by an active transport process using a transport protein!!! CoA is derived from a B vitamin (Thiamine) thiamine deficiency causes Beriberi
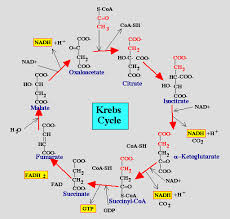
The citric acid cycle also called the Krebs cycle or the tricarboxylic acid (TCA) cycle, takes place within the mitochondrial matrix
The cycle oxidizes organic fuel derived form pyruvate generating 1 ATP, 3 NADH, 1 FADH2 per turn
The mitochondria are the major site of ATP production in the dark but the chloroplast is the site of ATP production in the light.In the bacteria the actual enzymes that carry out this cycle are in the cytosol because they don’t have mitochondria and the plasma membrane of bacteria contains the enzymes and complexes that carry out oxidative phosphorylation.
The citric acid cycle
- The citric acid cycle has eight steps each catalyzed by a specific enzyme.
- The acetyl group of acetyl CoA joins the cycle by combining with oxaloacetate forming citrate.
- The next seven steps decompose the citrate back to oxaloacetate making the process a cycle.
The NADH and FADH2 produced by the cycle relay electrons extracted from food to the electron transport chain.
Steps of Krebs cycle:
During oxidative phosphorylation chemiosmosis couples electron transport to ATP synthesis.
- Following glycolysis and the citric acid cycle, NADH and FADH2 account for most of the energy extracted from food
- These two electron carriers donate electrons to the electron transport chain which power ATP synthesis via oxidative phosphorylation.
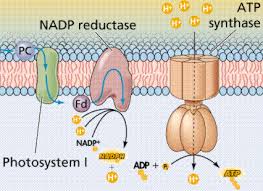
The pathway of electron transport
- The electron transport chain is in the cristae of the mitochondrion
- Most of the chain’s components are proteins, which exist in multiprotein complexes
- The carriers, alternate reduced and oxidized states, as they accept and donate electrons.
- Electrons drop in free energy as they go down the chain and are finally passed to O2 forming H2O
Electrons are transferred from NADH or FADH2 to the electron transport chain
Electrons are passed through a number of proteins including cytochromes (each with an iron atom) to O2.
The electron transport chain generate no ATP. The chain’s function is to break the large free–energy drop, from food to O2, into smaller steps that release energy in manageable amounts
Chemiosmosis: the energy-coupling mechanism
Electron transfer in the electron transport chain causes proteins to pump H+ from the mitochondrial matrix to the intermembrane space.H+ then moves back across the membrane passing through channels in ATP synthase.ATP synthase uses the exergonic flow of H+ to drive phosphorylation of ATP.This is an example of chemiosmosis the use of energy in H+ gradient to drive cellular work.The energy stored in a H+ gradient across a membrane couples the redox reactions of the electron transport chain to ATP synthesis.The H+ gradient is referred to as a proton – motive force, emphasizing its capacity to do work.
About40% of the energy in a glucose molecule is transferred to ATP during
cellular respiration,making about 38 ATP molecules.
Glucose oxidation ΔG = -686 kcal/mole.
ADP +Pi →ATP ΔG = 7,3 kcal/mol ⇒ 7,3x 38 = 277 kcal this make roughly
40 % of the total energy of Glucose.
Fermentation and anaerobic respiration enable cells to produce ATPwithout the use of oxygen
- Most cellular respiration requires O2 to produce ATP.
- Glycolysis can produce ATP with or without O2 (in aerobic or anaerobic conditions)
- In the absence of O2, glycolysis couples with fermentation or anaerobic respiration to produce ATP.
- Anaerobic respiration uses an electron transport chain with an electro – acceptor other than O2, for example sulfate.
- Fermentation uses phosphorylation instead of an electron transport chain to generate ATP.
Types of fermentation
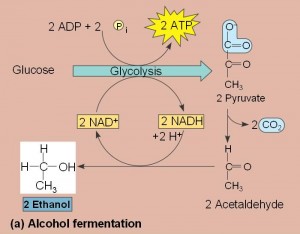
- Fermentation consists of glycolysis plus reactions that generates NAD+ which can be reused by glycolysis.
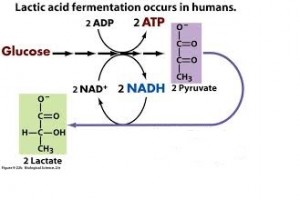
- Two common types are alcohol fermentation and lactic acid fermentation.
- In alcohol fermentation, pyruvate is converted to ethanol in two steps with the first releasing CO2.
- Alcohol fermentation by yeast is used in brewing, wine making and baking.
- In lactic acid fermentation pyruvate is reduced by NADH forming lactate as an end product with no release of CO2.
- Lactic acid fermentation by some fungi and bacteria is used to make cheese and yogurt.
- Human muscle cells use lactic acid fermentation to generate ATP when O2 is scarce.
Lactate is recycled to pyruvate in the liver (cori cycle) so in this pathway, we have 2 ATP produced. This is a much less efficient pathway.
Fermentation and aerobic respiration (compared)
- Both processes, use glycolysis to oxidize glucose and other organic fuels to pyruvate.
- The processes have different final electron acceptors, an organic molecule (such as pyruvate or acetaldehyde) in fermentation and O2 in cellular respiration.
- Cellular respiration produces 38 ATP per glucose molecule, fermentation 2 ATP per glucose molecule.
- Obligate anaerobes, carry out, fermentation or anaerobic respiration and can not survive in the presence of O2.
- Yeast and many bacteria are facultative anaerobes, meaning that they can survive using either fermentation or cellular respiration.
- In a facultative anaerobe pyruvate is a fork in the metabolic road that leads to two alternative catabolic routes.Human muscles cells are facultative anaerobs.
The evolutionary significance of Glycolysis
- Glycolysis occurs in nearly all organisms.
- Glycolysis probably, evolved in ancient prokaryotes before there was oxygen in the atmosphere.
- Glycolysis and the citric AC cycle, connect to many other metabolic pathways.
- Glycolysis and the citric AC cycle, are major intersections to various catabolic and anabolic pathways.
The versatility of catabolism
- Catabolic pathways, funnel electrons from many kinds of organic molecules into cellular respiration.
- Glycolysis accepts a wide range of carbohydrates.
- Proteins must be digested to amino acids, amino acids can feed glycolysis or the citric acid cycle.
- Fats are digested to glycerol (used in glycolysis) and fatty acids (used in generating acetyl COA).
- Fatty acids are broken down by beta oxidation and yield acetyl COA.
- An oxidized gram of fat produces more than twice as much ATP as an oxidized gram of carbohydrate.
Biosynthesis (anabolic pathway)
- The body uses small molecules to build other substances.
- These small molecules may come directly from food, from glycolysis or from the citric acid cycle.
Regulation of cellular respiration via feedback mechanisms
- Feedback inhibition is the most common mechanism for control.
- If ATP concentration begins to drop, respiration speeds up, when there is plenty of ATP, respiration slows down.
- Control of catabolism is based mainly on regulating the activity of enzymes at strategic points in the catabolic pathway.