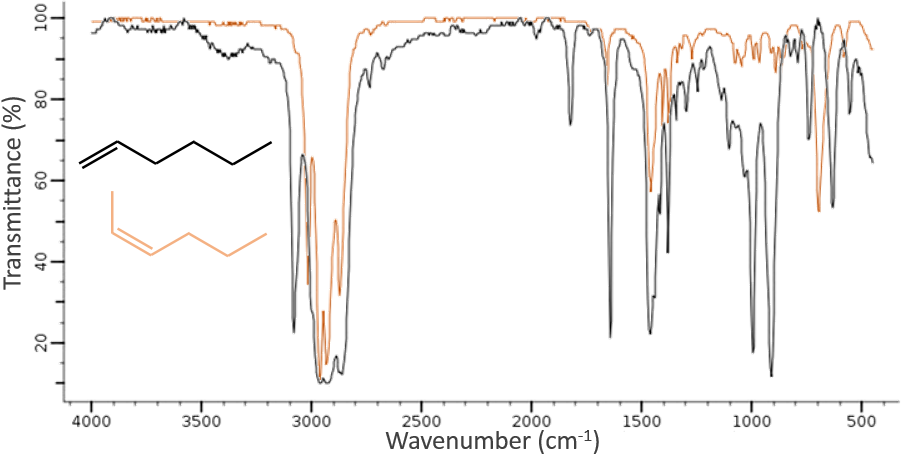
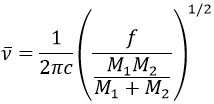The IR spectroscopy has a different goal than the UV/visible. It is one of the strongest methods to determine the structure of organic compounds. An IR spectrum is similar to the finger print of one molecule and the matching of peaks can tell us if a molecule is in the sample or not. The spectrum showed below is the one of the 1-hexene in black and of the cis-2-hexene in orange. The difference of position of the double liaison leads to a huge difference in the IR spectrum.
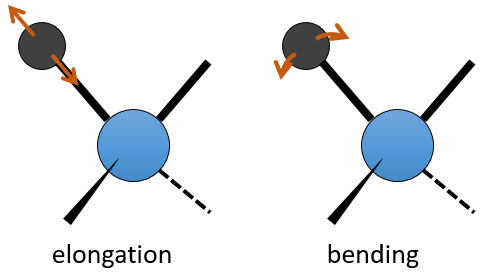
The UV/visible spectroscopy involves electronic transitions while the IR spectroscopy involves transitions between vibrational states. As for the UV/visible, we observe a series of bands, and not rays, because of the sublevel rotational states. There are two main modes of vibration: the elongation and the bending.
A liaison between two atoms has not a constant length. The atoms vibrate around their mass centre with a frequency that is characteristic of the pair of atoms. It is the elongation mode: a vibration in the axis of the liaison. However, the atoms are often bound to more than one other atom, modifying the frequency of the vibration and giving place to additional elongation processes.
The bending is a vibration out of the axis of the liaison. It leads to a variation of the angles between liaisons.
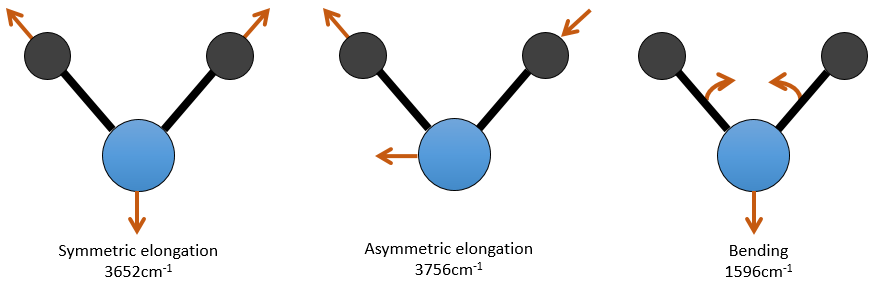
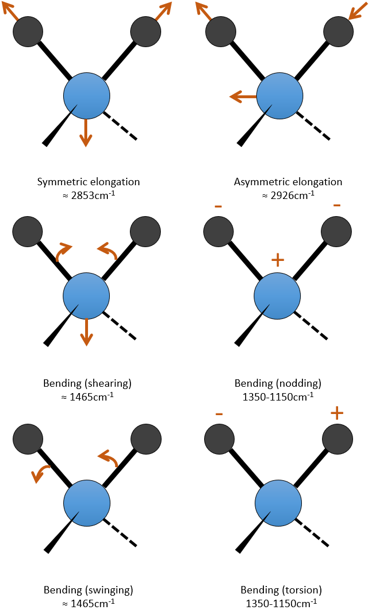
For a molecule of n atoms, the amount of degrees of liberty of the molecule is equal to the sum of the degrees of liberty of each atom. Each atom has 3 degrees of liberty corresponding to the 3 Cartesian coordinates required to describe its position in the molecule. The molecule has thus 3n degrees of liberty. Amongst the 3n, 3 are used to describe the translation modes and 3 are used to describe its rotation (2 if the molecule is linear). There are thus 3n-6 (or 3n-5) modes of vibration for each molecule. For instance, the water has 3 modes of vibration: 2 modes of elongation and 1 mode of bending.
One mode of elongation is symmetric (the centre of mass is displaced) and one is asymmetric. Each mode shows a specific vibration, with a given wavelength. The wavelengths of the elongation modes are very close to each other and the bending mode has a smaller wavenumber. The fact that the elongation modes and the bending modes are distant in terms of wavelength and that the bending modes have smaller wavelengths are generalities that we can find for any liaison.
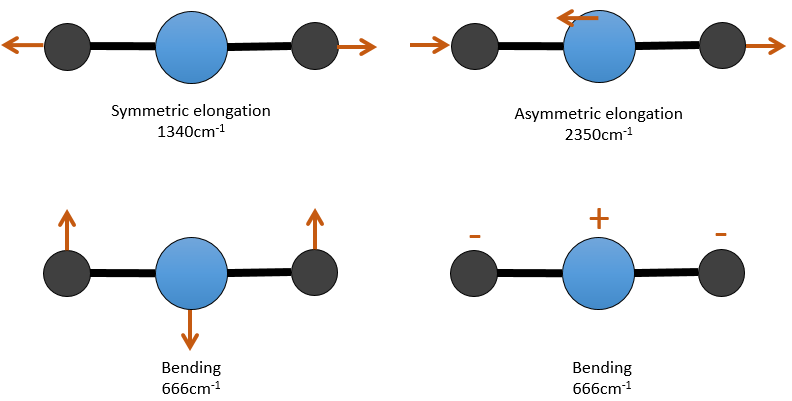
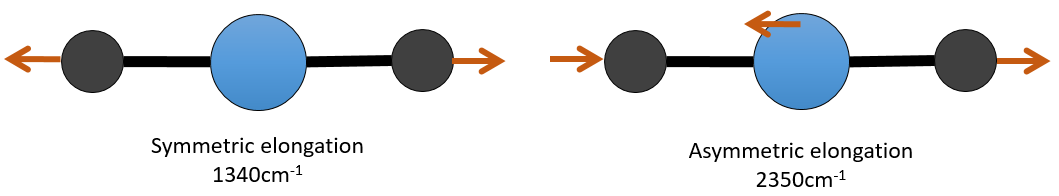
There are 3 atoms in the carbon dioxide but 4 modes of vibrations as the CO2 is linear (3n-5).
There is one symmetric and one asymmetric modes of elongation and two equivalent modes of bending (one in the x-y plane and one in the y-z). The bending modes have the same wavelength at 666cm-1. We say that those vibration modes are degenerated twice. Moreover, the symmetric elongation is inactive in IR because this mode of vibration does not induce any variation of the dipolar moment of the molecule.
In bigger molecules, it is rare to observe the exact number of modes of vibration (3n-6) because some modes come from combination from two vibrations or more, or are harmonics from strong modes of vibration. Those two effects increase the number of bands and other effects decrease this number
- bands that are too weak to be observed
- bands that are to close and that merge together
- degenerated bands
- inactive modes of vibration
- modes with wavenumbers out of the analysed range, usually between 4000 and 400cm-1.
It is possible to approximate the frequency of vibration of a given liaison with the law of Hooke that considers the liaison as a simple harmonic oscillator between two masses M1 and M2.
c is the speed of light and f is a constant that reflects the strength of the liaison, the value of which is approximately 5.105dyne/cm (1dyne=105N=105kg m s-2) for simple liaisons and two and three times this value for double and triple liaisons. f increases from left to right in the Mendeleev table. For instance, the law of Hooke gives a wavelength of 3040cm-1 for the C-H liaison. If we take a look to the vibration modes of CH2 inside a carbon chain, we find 6 modes of vibration (2 elongation modes and 4 bending modes).
Remember that the 3n-6 rule only applies to complete molecules. The elongation modes that we observe have frequencies that are a bit lower than the ones obtained by the law of Hooke (2926 and 2853cm-1 vs 3040cm-1) because of the environment of the C-H liaison that is not taken into account by Hooke.
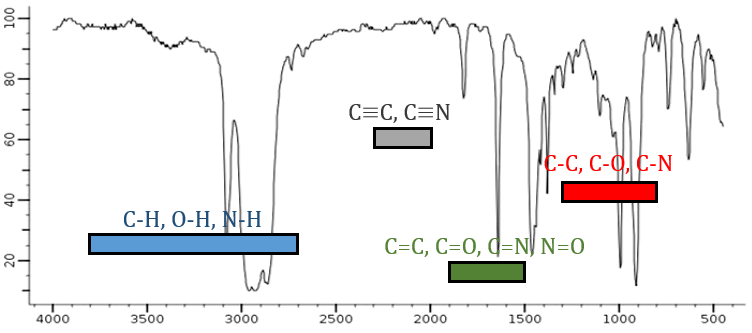
From the law of Hooke, it is obvious that heavy atoms vibrate at smaller frequencies. We can barely separate a IR spectrum in several regions depending on the pair of atoms involved in the liaison and the type of liaison:
| 3800-2700cm-1: C-H, O-H, N-H | 1900-1500cm-1: C=C, C=O, C=N, N=O |
| 2300-2000cm-1: C≡C, C≡N | 1300-800cm-1: C-C, C-O, C-N |
On the figure we can thus already deduce that there is no triple liaison (grey region) in our molecule (the 1-hexene from early), that there are some double liaisons (green region) and some simple liaisons (blue and red regions). However we cannot yet determine which peak corresponds to which vibration.
Coupled interactions
When two oscillators share a common atom, they rarely act as simple oscillators except if their vibrations modes are very different. The coupling between two modes of vibration produce two new modes of vibration with frequencies greater and smaller than the one in absence of interaction.
For instance, in the CO2, the asymmetric elongation (2350cm-1) and the symmetric elongation (1340cm-1) are the result of a coupling between the two C=O oscillations, one shortening when the other one elongates.
From what we have seen previously, one C=O should vibrate between 1900-1500 cm-1 but it is clearly not the case here. The coupling between the two C=O vibrations has for effect to displace the vibration towards larger frequencies (only the asymmetric is visible). The coupling becomes negligible when one or more carbons separate the liaisons. Two carbonyls separated by one or more carbons would show an absorption around 1725cm-1.
So the conditions to observe a coupling are that the vibrations share a common atom and have similar frequencies. However interactions are possible between fundamental vibrations and harmonic vibrations/vibrations of combination. Such coupling is called a resonance of Fermi. Still with the CO2, the symmetric elongation that is not active in IR but that can be observed in the Raman spectrum at 1340cm-1. In fact, there are two bands at 1286cm-1 and 1388cm-1 because of the coupling of the vibration with the harmonic of the bending modes (666cm-1). The first harmonic is thus at 1332cm-1 and interacts with the symmetric elongation mode at 1340cm-1.
Hydrogen bonds
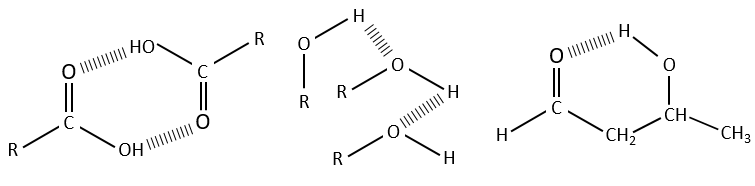
Hydrogen bonds decrease the frequencies of the involved liaisons and usually enlarge and enhance their bands. The importance of this effect depends on the strength of the H bond. A H bond is strong when it is in the exact direction of the lone pair of the electronegative atom. The strength of the bond also depends of the distance between the atoms, of the atom and if a cycle can be formed by the H bond.
The decrease of frequency varies from 300 to more than 500cm-1 if the H bond is intermolecular and from less than 100cm-1 to more than 300cm-1 if the bond is intramolecular. H bonds are often present between a molecule and the solvent. It is thus important to indicate the solvent and the concentration of the compound on a spectrum. The presence of water in the sample has a visible effect on spectra.
Analysis of a spectrum
An IR spectrum shows the transmittance as a function of the wavelength/wavenumber. The spectrum is to be read from top to bottom with bands dropping low in the spectrum.
An accurate analysis of an IR spectrum is not conceivable. The couplings, the absence of modes of vibration and the width of some bands make it difficult to determine the exact nature of the bands. However, as it has been said before, one IR spectrum is like the finger print of one compound and if one spectrum superimpose with the spectrum of a known molecule (with the same solvent, concentration and setup), the job is done. In other situations, an IR spectrum is used in combination with MS, UV and NMR spectra.
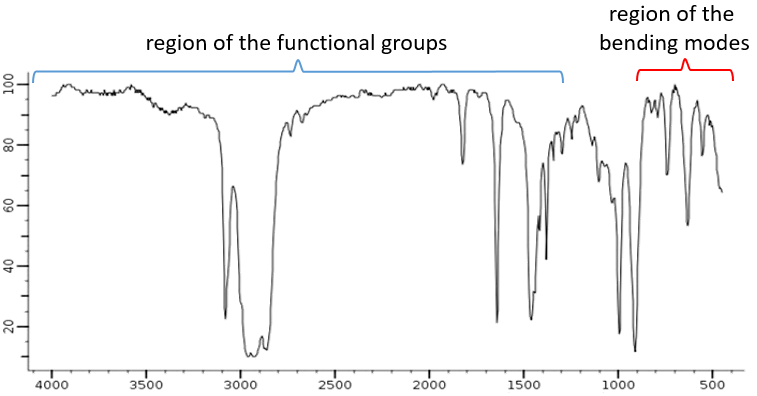
The spectrum, usually between 4000 and 400cm-1 can be split in two important regions to analyse: one between 4000 and 1300cm-1, called region of the functional groups, and one under 900cm-1 that shows the bending modes.
The elongation modes of functional groups with OH, NH, C=O can be found in the region of the functional groups. If this region is empty, we can assume that the molecule does not wear any of them. In rare situations, a weak and very wide band can be mistaken for an absence of band. Yet, be careful with low intensity bands that can also be harmonics or combination bands.
Characteristic absorbances
It would be too long to describe all the bands that can be observed on a IR spectrum. The goal here is more to give the very characteristic bands that can be easily spotted on the spectrum to have a general idea of what is in the molecule and what is not. In combination with a mass spectrum, it will be possible to determine the correct structure of the analysed molecule.
High frequencies vibrations
The region of the spectrum above 2850cm-1 is rich in information’s that can easily be studied. In this region we find the elongation bands of Y-H, Y=C, O, N. The bands are usually intense and are not hidden even if another band is nearby. The position of one band depends on the direct environment of the liaison. When necessary, we write this liaison (simple, double or triple) to demark =C-H, i.e. a hydrogen bond to a carbon with a double liaison, from –C-H for instance, i.e. a hydrogen bond to a carbon with a simple liaison.
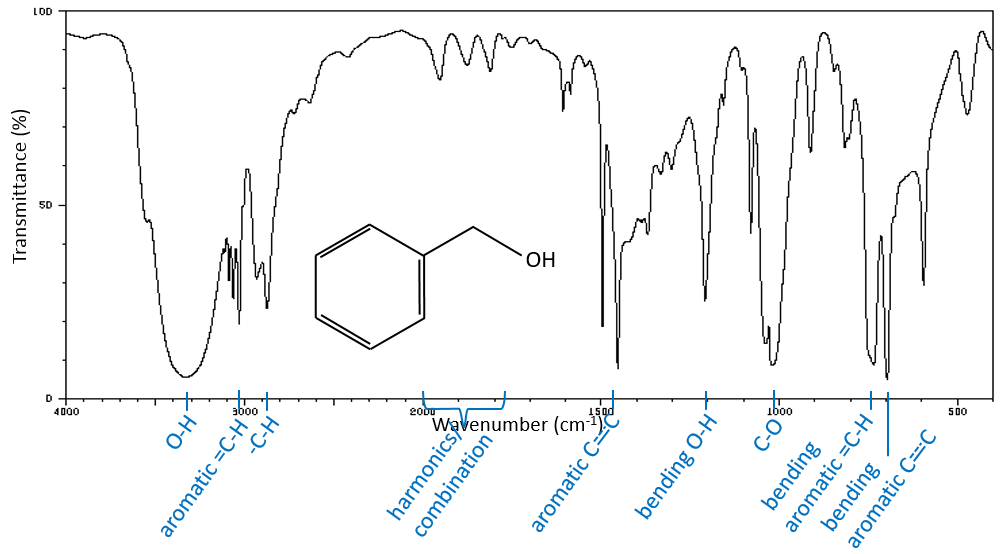
A first hint on the nature of the compound is found in the vicinity of 3000cm-1 where the C-H liaisons absorb. The difference of frequency between aromatic =C-H and aliphatic -C-H is small but is characteristic:
Methylic C-H are found as 2 bands at 2962cm-1 and 2872cm-1 and methylenic C-H are found a bit lower (2926 and 2853cm-1). =C-H are found slightly above 3000cm-1. It is often synonym of an aromatic but is can also simply be an alkene (or conjugated double liaisons). On the spectrum of the hexene, the peak at 3080cm-1 is characteristic of the double liaison and the peaks for the –C-H are hidden in the large cluster. Three small peaks are however visible around the values cited above for Methylic and methylenic –C-H.
It is thus easy to guess the presence of an aromatic cycle from the –C-H bands but it is not easy to determine if the =C-H absorbance band come from an aromatic or an alkene: both alkene =C-H and aromatic =C-H show an intense band in the low frequencies region, respectively between 1000-650cm-1 and 900-675cm-1. The aromatics (and heteroaromatics) will also show 3 peaks around 1600cm-1, 1500-1400cm-1 and 1300-1000cm-1 (note that conjugated alkene also show that kind of bands). Still in the spectrum of the hexene, the peak between 1300 and 1000cm-1 is not present and the bending peak is lower than 675cm-1.
The C-H bond of aldehydes vibrates at smaller wavenumbers, between 2830 and 2695cm-1 because the carbonyl takes electrons from the C-H and this liaison is thus longer, making it vibrate slower. The peak is not very intense and can be doubled because of a resonance of Fermi with the first harmonic of the bending mode at 1390cm-1.
≡C-H bond to triple liaisons are absorbing at frequencies above 3100cm-1, between 3333cm-1 (this one is easy to remember) and 3267cm-1 but are not the single species absorbing above 3100cm-1. We can also find –O-H and N-H elongation bands as well as H bonds. The ≡C-H bands are usually thinner than the bands for –O-H and –N-H and are intense.
-O-H elongation bands
The –O-H bands are large and intense, and very large in the case of carboxylic acids. In fact, those large bands are the result of the H bonds and the formation of dimers when the concentration of alcohol/acid are much diluted.
Free –O-H give small and thin bands between 3650 and 3584cm-1 for alcohols and around 3550cm-1 for acids but are easily erased by the large bands of H:O-H that are spreading over hundreds of cm-1 are way more intense. Those large bands should be centred between 3550 and 3200cm-1 (for the benzyl alcohol it is at 3320cm-1) for alcohols and 3000cm-1 for acids.
-N-H elongation bands
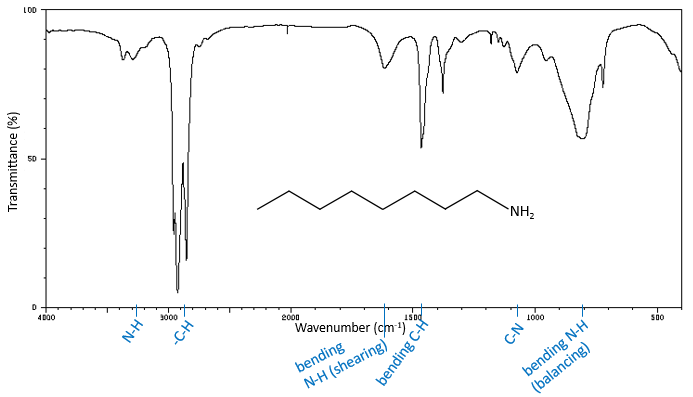
The high frequencies bands for aliphatic amines are quite weak between 3400 and 3250cm-1. There are two bands in the case of primary amines and 1 band in the case of secondary amine. The N-H elongation modes are slightly displaced towards larger frequencies if the amine is aromatic. The spectrum is different in the case of amine salts. Ammonium ions give a large and intense band of absorption between 3300 and 3030cm-1. The frequency decreases with the degree of the amine: primary amine: 3000-2800cm-1, secondary amines: 3000-2700cm-1, tertiary amine: 2700-2250cm-1).
In absence of H bond, the primary amide shows two bands around 3520 and 3400cm-1 while secondary amides show one band of absorbance between 3500 and 3400cm-1. In more concentrated solutions or in solids, H bonds displace the bands towards smaller frequencies, respectively around 3350 and 3180cm-1 for primary amides and multiple bands between 3330 and 3060cm-1 for secondary amides. The presence of multiple bands instead of one single band is explained by the formation of a dimmer of amides.
Middle range frequencies
Between 2850 and 900cm-1, we can find bands characteristic of elongation vibrations between two atoms other than H and some bending vibrations of Y-H that can confirm the observations from the high frequencies region of the IR spectrum. We will focus on the elongation bands.
The S-H liaisons produce a small band of absorption between 2600 and 2550cm-1. This band is lonely in this region of the spectrum but in diluted solutions the band can be to small to be detected.
C≡C shows a small band of absorbance between 2260 and 2100cm-1. This region is dedicated to triple liaisons (C≡N shows a band between 2260 and 2240cm-1) but harmonics and combination bands can be found in this region too. The bands of C=C are or middle or weak intensity between 1667 and 1640cm-1 for alkenes that are not conjugated. Those alkenes are found between 1650 and 1600cm-1. The cumulated alkenes (c=c=c) absorb between 2000 and 1900cm-1.
Elongation vibrations of C=O
The absence of absorbance between 1870 and 1540cm-1 is synonym of the absence of carbonyl. In this region, the position of the peak depend on the conjugation of the carbonyl and of the substituents (which substituent and its mass) and the possible H bonds.
An aliphatic ketone is found at 1715cm-1. Modifications of the environment of the ketone can induce either an increase of a decrease of the wavenumber of the peak that depends on the predominance of the inductive or of the resonance effect. The inductive effect reduces the length of the C=O liaison, leading to the increase of its frequency of vibration. A resonance increases the length of the C=O liaison, displacing the peak towards the C-O peak that is at a lower frequency. For instance, Cl has a predominant inductive effect and the peak of the C=O is at 1815-1785cm-1. The carbonyl of a primary amide vibrates at 1695-1650cm-1 because the resonance effect is predominant. There are two bands for primary amides and only one for the other ones. Carbonyls conjugated with alkenes have peaks of absorption at smaller wavenumbers than the aliphatic ketone between 1685 and 1666cm-1. An aldehyde absorbs a bit higher than the ketone (1740-1720cm-1). An acid absorbs strongly (way more intensely than a ketone) around 1760cm-1 but H bonds in dimmers of acids can displace the peak towards smaller frequencies (1720-1706cm-1). Esters absorb at 1750-1735cm-1. An acyl halide shows a strong absorption between 1815 and 1785cm-1.
H bonds decrease the frequency of vibration of the carbonyls. The effect is small for intermolecular H bonds (a dozen of cm-1) but can be important for intramolecular H bonds.
Elongation vibrations of C-O
In the middle range spectrum, between 1300 and 900cm-1 the spectrum is usually complicated. It is the “digital print” of the compound where we can find the bands of alcohols C-C-O that should be accompanied with O-H bands at large frequencies.
The C-O liaison of alcohols shows an intense band due to the vibrations of elongation between 1260 and 1000cm-1. This mode of vibration is usually coupled with the next carbon, as an asymmetric elongation C-C-O. The C-O-C of ethers and epoxides systems can also be found in this region of the spectrum, between 1150 and 1086cm-1 (for its asymmetric elongation, the symmetric being very weak because it does not modify the dipolar moment) as an intense band or several bands if the nearby carbons are ramified. If one side is an aryl group, we observe one intense band (asymmetric elongation) at 1275-1200cm-1 and one intense band (symmetric elongation) at 1075-1020cm-1). Finally, the vinyl ethers show similar bands (1225-1200cm-1 and 1075-1020cm-1).
Acids give two bands of in unequal intensities, in addition of the one of C=O, coming from an interaction between the elongation mode of C-O and the bending mode of O-H. We talk about the bands of C-O-H. The most intense band between 1315 and 1280cm-1 is called the elongation band C-O. The second band, between 1440 and 1395cm-1 is the band called the band of bending O-H. This band falls in the same region than the shearing mode of CH2. The carboxylate also gives two bands of unequal intensities, the most intense one between 1650 and 1550cm-1 and the other one around 1400cm-1.
A particularity of the esters is their intense band of absorption at the place of the elongation mode of the ketones. This band is not the band of C=O, that is displaced towards larger frequencies but one of the two bands of C-O. The C=O band is indeed between 1750 and 1735cm-1 and 20cm-1 lower if the carbonyl is conjugated. The C-O vibration is coupled with both sides of the liaison, leading to two bands of absorption. The most intense one is C-C(=O)-O giving a peak between 1300 and 1000cm-1. Esters of aromatic acids absorb strongly between 1310 and 1250cm-1. The other band between 1111 and 1031cm-1 comes from the coupling O-C-C. Its frequency depends on the substituent of the ester. Esters of primary alcohols are at the lowest frequencies (1064-1031cm-1) while esters of secondary alcohols and of aromatics absorb around 1100cm-1and 1111cm-1 respectively.
Elongation vibrations of NO
Nitro groups have a symmetric and an asymmetric elongation mode of vibration. The asymmetric mode gives an intense band between 1661 and 1499cm-1. The symmetric mode gives a band between 1389 and 1259cm-1. The wavenumbers of both bands depend on the substituent of the nitro group. In the case of an alkane, the bands should be observed around 1550 and 1372cm-1 respectively. An electronegative group on the alpha carbon increases the frequency while a conjugation decreases the frequency.
Nitrates show 2 intense bands of elongation for N=O (one asymmetric and one symmetric) between 1300-1255cm-1 and 1660-1625cm-1. Nitrites have two bands as well, one for the cis (1625-1610cm-1) and one for the trans isomer (1680-1650cm-1).
Nitroso compounds show an absorption band between 1585 and 1539cm-1.
Elongation modes of C=S
The elongation modes of C-S are found in the low frequencies range and the C=S slightly above this region, between 1250 and 1020cm-1. This band is less intense than the one of a C=O because it is less polar.
Elongation modes of S=O
The S=O liaisons produce intense bands in general. The S=O liaison of sulfoxides vibrates between 1070 and 1030cm-1. Sulfones show two bands of absorption between 1350-1300cm-1 and 1160-1120cm-1. The bands are displaced towards larger frequencies in the case of a sulfonyl chloride (1410-1380cm-1 and 1204-1177cm-1) or of a sulfonamide (1370-1335cm-1 and 1170-1155cm-1).
Bending in the middle range region
Several liaisons with elongation modes of high frequency, typically Y-H liaisons, have bending modes that fall in the same region than other elongation modes (Y-Z). They complicate the reading of the spectrum but can also confirm peaks of the high frequencies region.
C-H bending modes
The bending modes of the methylenic C-H liaisons have been seen at the beginning of this chapter. One mode is in the low frequencies region at 720cm-1 and the three other are in the middle range region. The shearing mode has an almost fixed wavenumber of 1465cm-1 but the two last modes (nod and torsion) give peaks between 1350 and 1150cm-1. In a cycle, those frequencies are slightly decreased. Methylic C-H have two bending modes: one where the three H oscillate in phase and one wherein one H is not in phase. The symmetric mode (in phase) gives a peak at 1375cm-1 and the asymmetric mode at 1450cm-1, almost at the same frequency than the shearing mode of a methylenic C-H. =C-H of alkenes absorb at 1415cm-1 due to a shearing mode. Aromatics have a mode of bending out of the plane that give intense peaks in the low frequencies region and a bending mode in the plane give peaks between 1300 and 1000cm-1.
Bending O-H
Peaks of alcohols are not very characteristic and are in the region between 1420 and 1330cm-1. If there is a proton on the carbon wearing the –OH (i.e. primary and secondary alcohols), there is a coupling that gives two bands around 1420 and 1330cm-1. Tertiary alcohols give only one peak. In the case of the acids, there is an interaction between the elongation mode of C-O and the bending mode of O-H (discussed in the C-O elongation modes section). The C-O-H bending mode should be found between 1440cm-1 and 1395cm-1.
Bending N-H
Primary amines have a bending mode that gives a peak of average to high intensity between 1650cm-1 and 1580cm-1. The bending mode of secondary amines is hardly detectable except for aromatic secondary amines that absorb around 1515cm-1. In the case of amides, the N-H bending mode should be observed by a band between 1655 and 1590cm-1 with an intensity way smaller than the intensity of the C=O peak. As C=O absorbs in the same region, the peaks of the elongation C=O and of the bending N-H can merge.
Low frequencies region
In this region we find mostly the bending modes but some elongation modes are also present. In my opinion, this region is less interesting than the high and middle frequencies regions and is pretty hard to read because several modes have large ranges of possible frequencies that overlap with other modes. The most characteristic peaks are the peaks for dimmers and for aromatics. Between 900 and 650cm-1, a large and intense band is characteristic of a dimmer of acid, amide or amine. In the same region, the absence of intense peaks indicates that the molecule is not an aromatic.
Bending modes of CH
The peak of the swinging mode of methylenic -C-H at 720cm-1 has a low intensity or is not visible. A very intense band (usually the most intense of the spectrum) is visible between 1000 and 650cm-1 if an alkene is present in the molecule, coming from a bending mode out of the plane. It is usually this kind of bending out of the plane that gives intense bands in the low frequencies region. A very intense peak can thus be observed for aromatics between 900 and 650cm-1. The alkynes give a thick and intense peak at lower frequencies, between 700 and 610cm-1.
Other bending modes
The bending mode of alcohols gives a thick peak between 769cm-1 and 650cm-1 in addition of the bending modes of the middle frequencies region. Dimers of acids give a very broad band (not as large as the band in high frequencies that the acids give but it is characteristic) of average intensity centred near 920cm-1. Amides also give a thick band of average intensity but between 800 and 666cm-1, again due to a bending out of the plane. This bending is stronger and at higher frequencies for amines (909-666cm-1).
The bending out of the plane of the amines and amides generate one average/intense band respectively between 909-666cm-1 and 800-666cm-1. Nitro groups seem to show a bending mode between 763 and 690cm-1.
Elongation modes in the low frequencies region
A band of elongation of N-O appears between 870-833cm-1 from nitro groups and between 850-750cm-1 from nitrites. C-S absorbs between 700 and 600cm-1.
Finally, halogens have their elongations modes in the low frequencies. In an aliphatic chain, C-Cl absorbs between 850 and 550cm-1, C-Br between 690 and 515cm-1, C-I between 600 and 500cm-1 and C-F between 1400 and 730cm-1. The band of C-F is strong and large. If the substituent is an aromatic, the bands are displaced towards larger frequencies, above 1000cm-1 in the case of chlorobenzenes.