The word protein comes from the Greek word proteos, which means first. Proteins are indeed one essential element of the life. They are well defined in composition, size and shape and each one has a very precise role: transport, defence, hormones, … Some have also an exotic role. For instance some proteins prevent the blood of fish to freeze. We can however sort the proteins in three main classes: fibrous, globular and membrane proteins. The fibrous proteins are stretched out and fragile. Two examples are the keratin and the collagen. The keratin is one tough and insoluble fibrous structural proteins that composes hair, wool, horns, nails, claws, etc. The toughness of the keratin is rivalled only by the chitin in biological materials. They are not only localised in the hard parts of animals but are found in all epithelial cells, i.e. cells that cover the external surfaces of organisms and internal surfaces of organs to reinforce their structure. Horns, claws, nails … are produced by epithelial cells adapted to growing an abundance of keratin and then dying as individual cells while leaving the keratin to help form a structure valuable to the whole animal. The collagen is the main structural protein in the extracellular space, making it the most abundant protein in mammals. They form bones, tendons, cartilage in function of the local degree of mineralization. Membrane proteins are found in membranes and serve as receptors or provide channels to transport ions or molecules from one side of the membrane to the other side. Globular protein are basically the non-fibrous proteins and have diverse functions (example: haemoglobin, lysozyme). All the enzymes are globular proteins.
One common point between all the proteins is that they are insoluble in organic solvents. Thanks to that, we were able to determine their (crystalline) structure and composition. For instance, in the bones we found the collagen by crystallisation. We can destroy the protein to determine the amino acids it is made of: glycol. In the silk, we discovered the fibroin, composed of serine.
Amino acids (AA)
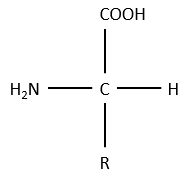
The general structure of amino acids is
where R is the group that makes the distinction between all the different amino acids (with one exception) .They are zwitterions: they wear an acid group (COOH) and a basic group (NH2). The central carbon is chiral and the AA can be L or D. 20 AA are found in the proteins (over 23 AA).
The AA are usually written by an abbreviation of 3 letters are sorted in function of their functional group R
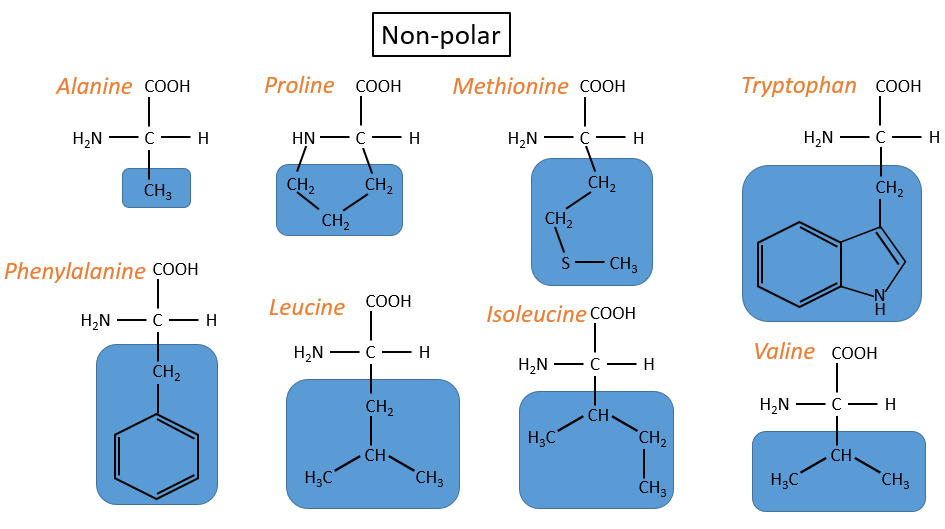
- non-polar hydrophobic: no interaction with water
- proline: its R is cyclic and binds to the amine group of the AA. It forbids the rotation of the AA.
- methionine: it possesses a sulphur atom
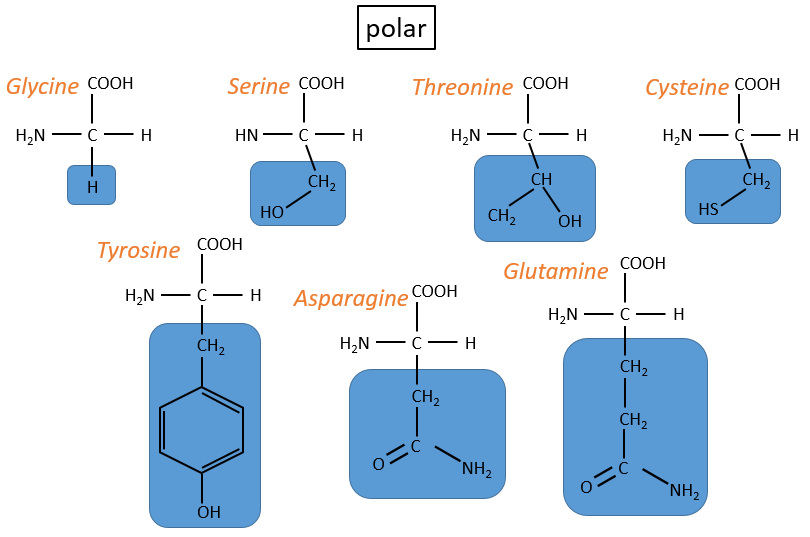
- neutral polar: may react with the solvent. They are polar because R is very small
- cysteine: they bind together via S bonds
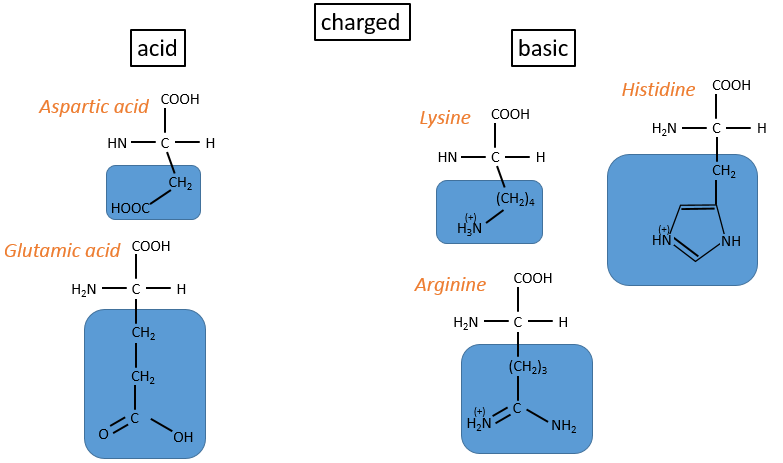
- charged polar
- acids: with COOH
- basic: with amine derivatives
The isoleucine and the threonine have one asymmetric carbon. Only the L are natural.
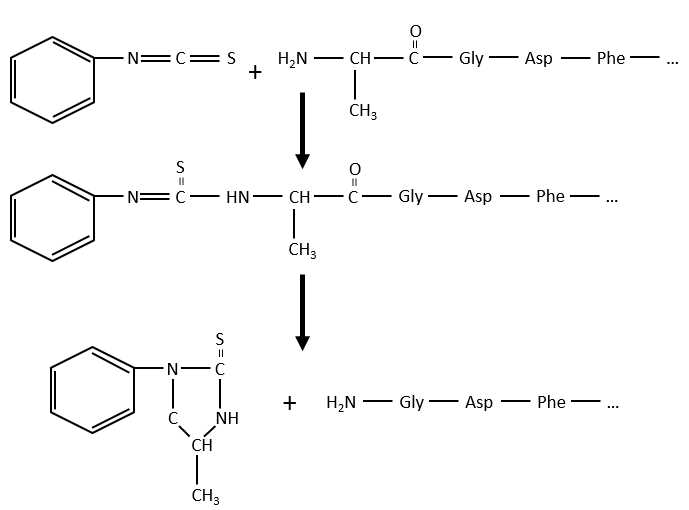
As zwitterions, the amino acids have an isoelectric point that depends on the nature of R. We can easily determine the composition of one protein thanks to this property: once hydrolysed, the amino acids that compose the protein can be separated by chromatography or electrophoresis to determine their proportions. There is also a way to determine the last amino acid of the protein (ended by NH2): prior to hydrolysis, we use the reactant of Sanger (1-fluoro-2-4-dinitrobenzene). This coloured group is fixed on the amine and the bond cannot be hydrolysed. We can thus identify it after the chromatography. Another method is the degradation of Edman. In this case a phenyl isothiocyanate reacts with the amino-terminal residue to form a thioamide. The next peptide bond is weakened and can be cleaved without hydrolysis because of the formation of a cycle.
The process can be repeated several times to determine the initial amino-terminal sequence.
The interactions between the AA of the protein with the other AA and with the solvent are responsible for the structure of the protein.