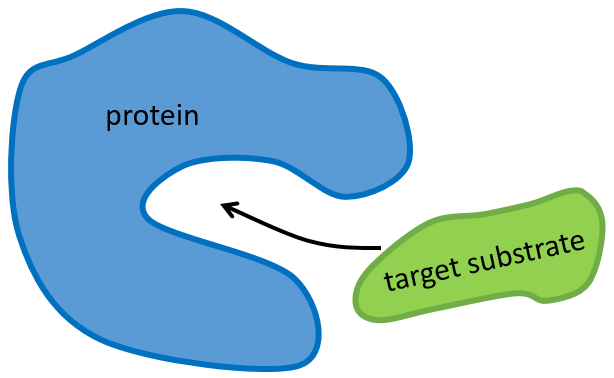
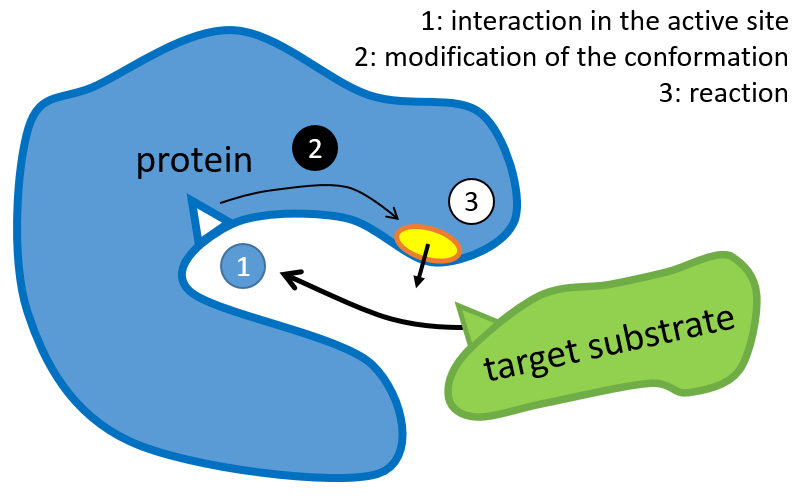
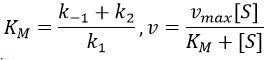The structure of the protein is obtained during the formation of the protein. The structure of the protein is necessary for its function: some active sites are present on the proteins. Those sites are highly specific to one target substrate. Other substrates cannot reach the active site.
The rest of the molecule is mostly there to ensure the specificity of the reaction or a modification of the conformation of the protein once an active site is activated (to submit a signal for instance). The peptide chain can also help the reaction to occur by the application of a pressure on one specific liaison of the target molecule.
A protein is denaturised when its conformation is modified. Consequently, its function is lost. It can be done through heating (~60°C), a strong base/acid, … It is frequent that under those conditions the protein precipitates.
The determination of the spatial configuration of the ribonuclease was done in 1950.
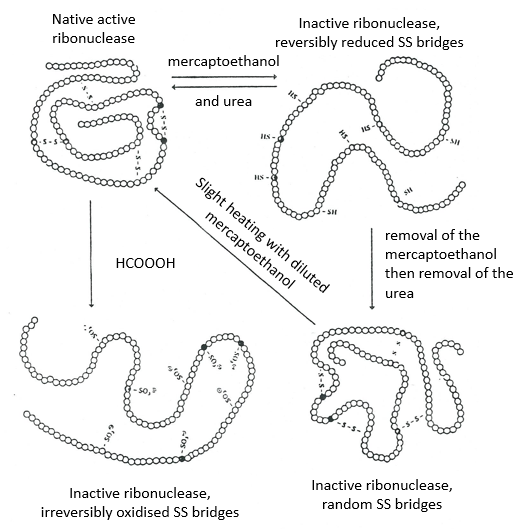
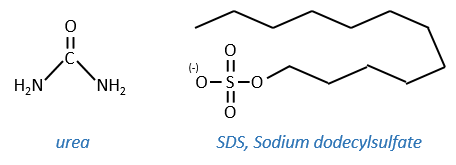
This protein has 4 disulphuric bonds (8 cysteines). Anfinsin placed the protein in a solution with mercaptoethanol and urea (NH2-CO-NH2). The mercaptoethanol cleaves the S-S bonds while the urea forms H bonds with the protein. The activity of the protein decreased by 99%. The normal conformation of the protein could be reobtained by placing the solution is a dialyse bag: placing the bag in a clear solution without urea and without mercaptoethanol, these molecules move out of the bag while the protein remains inside. Its activity comes back to 100%. It is interesting to note that the S bonds were all formed at the right place (amongst 105 possibilities). The mechanism is too fast to be try and errors.
If we removed only the mercaptoethanol (the solution outside the dialyse bag has already urea in it), the S bonds are done but at the wrong places.
All the proteins don’t get back to their functional form after a denaturation. They are build sequentially during their synthesis and the interactions between the chains are done before the complete synthesis of the protein. It is thus unusual that the sequence that was made first binds with one of the last sequence. However, after a denaturation, any part of the peptide chain can bind with any sequence of the chain, no matter the order of formation.
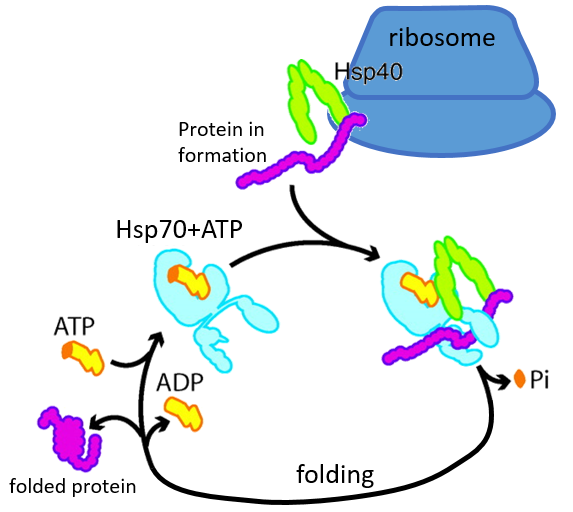
Some proteins are there to help to the correct deployment of other proteins. They are called heat stock proteins (hsp70 and hsp60): after an increase of temperature, several hsp70 recognizes the hydrophobic parts of peptide chains and bind with the protein. It helps the protein to reach the correct conformation and the hsp70 leave the protein next. The hsp60 acts on proteins that are already wrongly deployed and correct their structure.
Determination of the structure of proteins
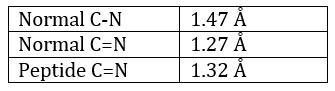
The method is based on the diffraction of X rays. From crystals of the proteins we obtain a distribution of the atoms. Payling and Corey analysed the interatomic distances between the different atoms in the amino acids. They found out that the peptide bond has a length between the length of a liaison C-N and a liaison C=N.
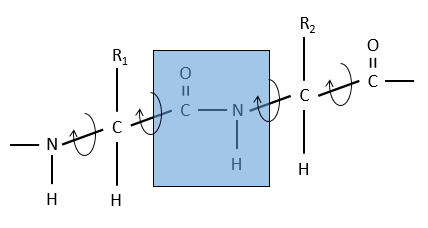
The reason is the resonance with the carbonyl. The peptide bond is thus rigid, almost always trans, but the liaisons around the peptide bond can rotate.
Secondary structure
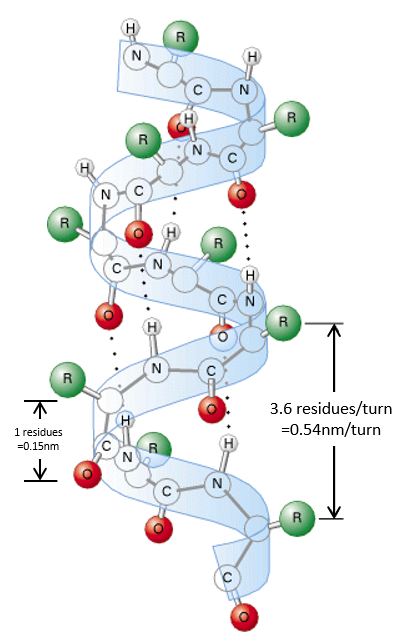
The secondary structures of a peptide chain are small “motifs” due to the interaction between nearby AA. The first structure, the α helix, was proposed in 1951. The distance between spires of the helix is 0.54nm and the length of one AA is 0.15nm. There are thus approximatively 3.6 AA by spire. All the R groups are at the outside of the helix, which is always L. There are H bonds between AA distant of 3-4 AA
All the AA cannot take place in helices. The structure is compact and AA with voluminous R group (for instance with an aromatic in R) would deform the helix.
The proline cannot be part of a helix too: its R is covalently bound to the nitrogen of the peptide bond. The liaison is thus particularly rigid and cannot be placed in one helix.
A third exclusion of the helices is charged amino acids. Imagine a helix with several –COOH groups. The structure is stable in neutral conditions but if the pH changes, there can be a lot of nearby COO-, what is unstable.
We find a lot of α helices in fibrous proteins such as the keratin α (found in the hairs). The hydrophobic AA form helices and we find them in lipid membranes.
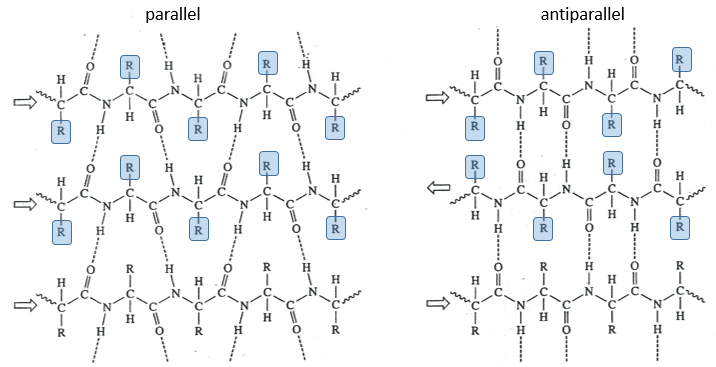
The β sheet structure is a stretched out structure of the peptide chain. The chains place themselves in parallel or in antiparallel to form a compact and rigid structure.
The chains are bond by H bonds and the distance between the chains is 0.35nm. The R groups of the AA have to be small to fit in the space between the chains. Even smaller in the antiparallel β sheets because the R groups are head to head with the R groups of the other chains. In the parallel β sheets, the R groups face one hydrogen. This kind of structure is also largely found in fibrous proteins such as the fibroin (in the silk) or the β-keratin (in feather).
There are also β helices, formed of β sheets arranged in one helix, but these are less frequent.
Tertiary structure
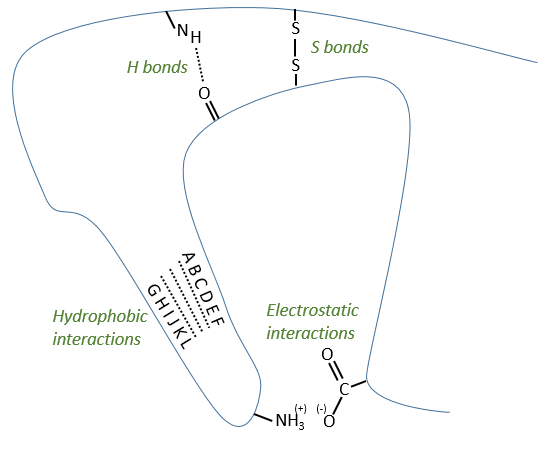
The tertiary structure is the result of long distance interactions. In a general way we represent helices by tubes/helices and β structures by arrows. The extremities of the chain are indicated by NH3+ and COO–.
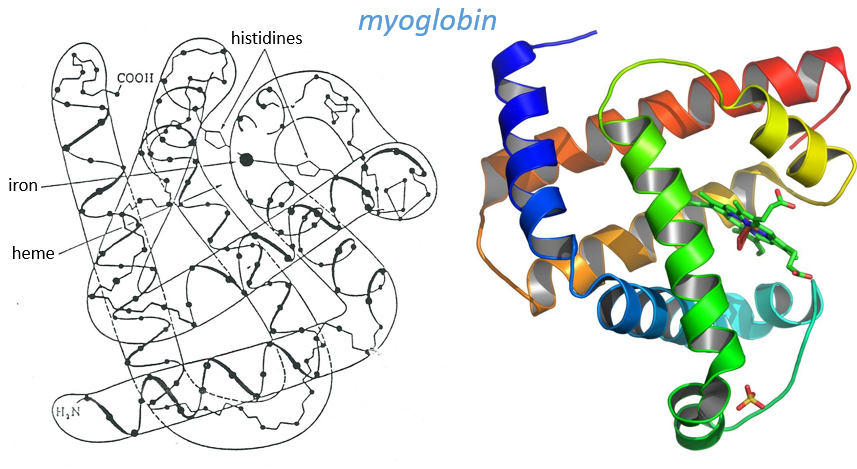
Kendraw determined the tertiary structure of the myoglobin of cachalots. To do so he marked with heavy atoms some known sequences of the molecules.
The structure is compact and possesses 8 helices of variable sizes. The groups inside the protein are nonpolar at the exception of two histidines. The AA in the helices may vary for different species but the angles remain the same.
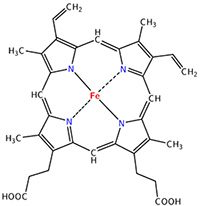
The heme group is the one that fixes the oxygen to supply the muscles. The heme is a tetrapyrol on which one Fe2+ is fixed at the centre.
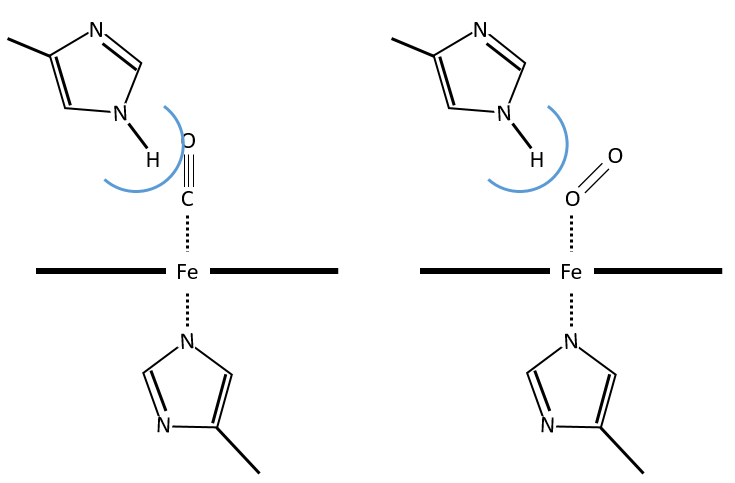
The iron has 6 liaisons and the two last are perpendicular to the plane made by the 4 other liaisons and bind with the two histidine’s. The heme is in the hydrophobic part of the protein because otherwise the iron would be oxidised by the blood. O2 fixes on the iron instead of one histidine. Yet, the presence of the histidine is very important: oxygen can easily bind on the heme but CO has an affinity for the heme 2500 larger than the one of the O2 without the presence of histidine’s. So if there are a few molecules of CO in the air, they would bind to the myoglobin and stay on it for a long time, forbidding oxygen to reach the muscles. The way to heal such an asphyxia is to place the person in a hyperbaric box full of oxygen (and obviously without CO) where we play on the equilibrium to replace the CO by O2. If there are 5000 molecules of O2 for each CO in the air, then the O2 is more susceptible to bind with the haem. Fortunately, histidine’s are close to the iron and prevents sterically the CO to bind correctly with it. The oxygen is not obstructed by the histidine because the angle of liaison is not the same (see the figure below). Considering the histidine, the affinity for the CO drops down to 200 time the one of the oxygen.
Types of liaisons in the tertiary structure:
- hydrophobic interactions
- electrostatic liaisons
- S bonds (cysteines)
- H bonds
Denaturation of the tertiary structure
- by heating: the heat is enough to break weak liaisons such as the hydrophobic interactions. As they break, the structure shambles and the hydrophobic parts enter in contact with the aqueous environment. As a result, the protein precipitates
- by urea: the urea breaks the H bonds
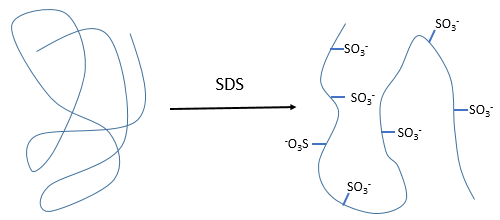
- by detergent: it denatures and solubilises the protein. For instance the SDS (sodium dodecylsulfate).
The hydrophobic chain of the detergent interacts with hydrophobic sequences of proteins. At this point the SO3– is transferred on the protein, generating some hydrophilic zones that modify the structure of the protein.
Quaternary structure
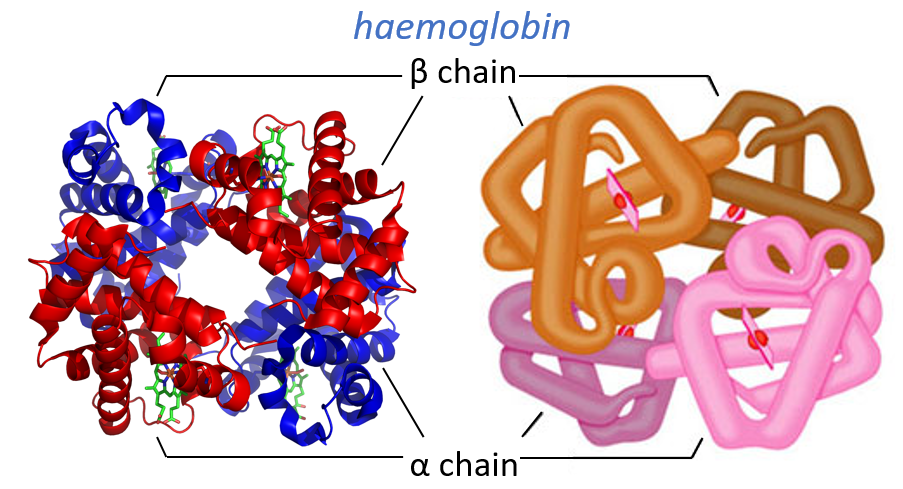
Some proteins aggregate together to form a bigger structure. The parts of this structure are called protomers and are only active if they are together. For instance, the haemoglobin is composed of four protomers, two α and two β.
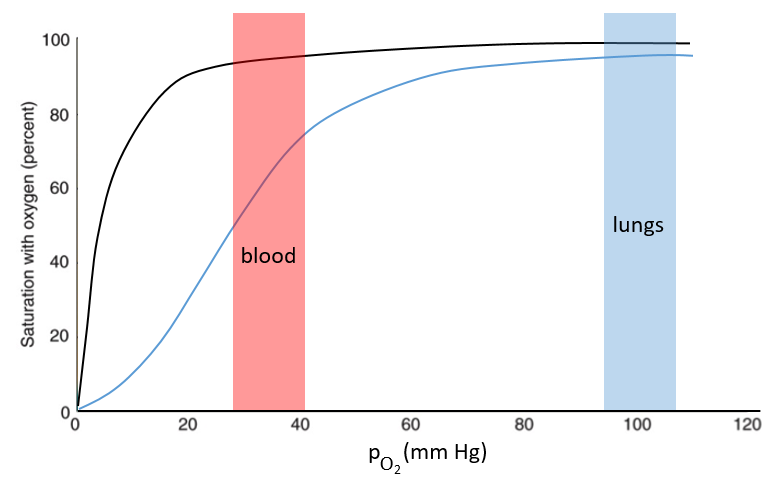
The function of the haemoglobin is to transport the oxygen in the blood and to transfer it to the myoglobin at the muscles. By the way, the protomers are alike with the myoglobin but only in the tertiary structure. The primary structures are very different except at the corners. To give an idea of the role of the haemoglobin, without them there would be 5ml of O2 by litre of blood. With them in the blood, there are 250ml of O2 by litre of blood. Another difference between the myoglobin and the haemoglobin is their curve of fixation of the oxygen. i.e. the percentage of saturation of the protein as a function of the partial pressure in O2.
The curves have similar shapes but the one of the haemoglobin is shifted in larger pressures of O2 In other words, and as expected, the two proteins don’t fix the oxygen at the same place. The partial pressure is high in the lungs and is around 40mm Hg in the muscles and tissues. The two proteins are saturated in oxygen at high pressures but the myoglobin has a rate of saturation that increases much earlier than the one of the haemoglobin. As the myoglobin is only found in the muscles, the haemoglobin fixes oxygen in the lungs and transports it to the muscles. In the muscles, the myoglobin has approximatively 40% more affinity for the oxygen than the haemoglobin. Haemoglobin drops some of the oxygen that is collected to the myoglobin.
The difference of behaviour is explained by the structure of the haemoglobin. The fixation of the first oxygen is difficult and induces a modification of the conformation of the protein through the rotation of two protomers. It opens up the way to a larger central cavity of the protein and the oxygen can now enter more easily. The form without oxygen is called deoxyhaemoglobin and the form with oxygen is called oxyhaemoglobin.
In the cavity, there are some charged amino acids that may enter in interaction with diphosphoglycerate (DPG), a molecule present near muscles and tissues but not in the lungs. With the DPG on the haemoglobin, the curve of fixation of oxygen goes down. By selectively binding to deoxyhaemoglobin, BPG makes it harder for oxygen to bind haemoglobin and more likely to be released to adjacent tissues. The concentration of DPG depends upon the location in the body but we find it essentially in the muscles and in the blood, what explains the difference of the curves of the myoglobin and of the haemoglobin. When we go in altitude, the pressure decreases significantly and we also produce more DPG. It allows the muscles to work better because there is a better transfer of oxygen from the haemoglobin. The DPG is not immediately degraded when we come back to a normal altitude and the muscles continue to be more efficient for a period of time. It is why athletes are often going to the mountain to practice before important events.
The Bohr effect
Haemoglobin can also fix CO2 and protons (both are products of the muscles). Their fixation favours the exchange of oxygen at low pressures. Basically, when we do sport, or run away from a danger, muscles work, produce CO2 and protons. The body uses those products as a signal to ask for more oxygen to maintain the effort. It is an allosteric effect: the activity of the protein is modified by agents that act on a different place of the protein than the active site. The CO2 is fixed on the terminal NH2 of the peptide chains while the protons are fixed on histidines.
Enzymes
Enzymes catalyse chemical reactions with a huge specificity. Usually there is only one target substrate but sometimes other molecules can take place in the active site and block it. The names of the enzymes are often related to the target substrate. For instance, the enzymes responsible for the cleavage of RNA is the RNAase, of lipids a lipidase, of amino acids a protease.
The activity of enzymes is modulated by several factors
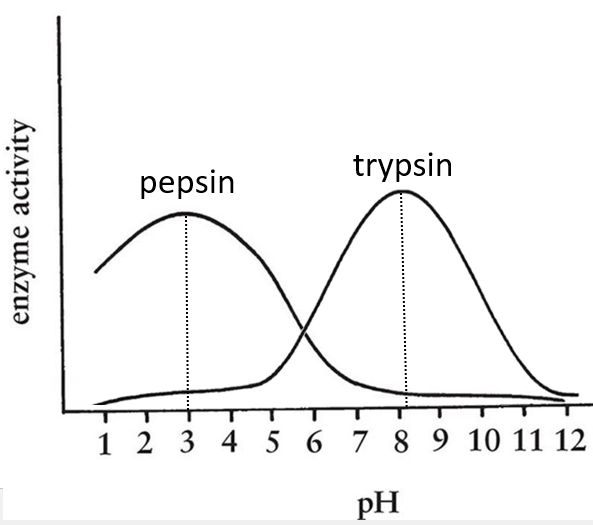
- pH: there is usually a maximum of activity at a given pH. For instance the pepsin and the trypsin are two enzymes that are respectively active in the stomach and in the intestine. It is thus normal that the optimum of pH of the pepsin is much lower (more acidic, pH»3) than the one of the trypsin (pH»8).
- cofactors: they can be ions or coenzymes and their presence will affect the activity of the enzyme. As for the pH, there can be a maximum of activity depending on the concentration of the cofactor.
- temperature: chemical reactions obey to the law of Arrhenius
The temperature increases the speed of reaction but if it increases too much the enzyme is denatured. There is thus also an optimum of temperature. In the body, this optimum is usually around 37°C.
- saturation: the concentration of enzyme is often small in comparison to the concentration of substrate so it limits the maximum speed of reaction. If we increase the concentration of enzyme, the maximum speed increases but it is only true up to a given point. After this point, we can increase the concentration of enzyme but the speed will not change. It is translated by the constant of Michaelis-Menten (see the chapter on kinetics).
A small KM means that the enzyme reaches its full speed for lower concentrations. Half of the maximum speed is reached when the concentration of substrate equals KM.